Israel’s clinical trial for a COVID-19 vaccine candidate developed by the government-run Israel Institute for Biological Research (IIBR) kicked off on Sunday morning with the first Israeli volunteer, 26-year-old Segev Harel, getting the injected dose at the Sheba Medical Center outside Tel Aviv.
The hospital released a short video of Harel on Saturday night saying he was feeling healthy and feeling confident. He also said his participation was “a great privilege.”
SEE ALSO: Israeli Bio-Defense Lab Gets Okay To Begin Clinical Trial For New COVID-19 Vaccine
“So many people have been harmed by the coronavirus, from a health perspective, mentally, and mainly economically, and if this is the little contribution I can make – to participate in this [trial] and bring hope that we are on a path to end this pandemic – then I’ve done my part,” said Harel, a resident of Kibbutz Sde Nehemia in northern Israel and an undergraduate student at Ruppin College in Netanya.
Harel added that he was “certain” everything will go well.
Israeli Prime Minister Benjamin Netanyahu and Defense Minister Benny Gantz, also the alternate prime minister, met with Harel at the hospital and praised the start of the trial.

“The true exit from the coronavirus crisis is in the development of vaccines,” said Netanyahu in reference to Israel’s gradual emergence from a second nationwide lockdown that is currently underway.
“Therefore, this is a very important day, a day that gives a shot of encouragement. We just met Segev, a 26-year-old Israeli man, who volunteered to be the first on the frontline and receive the experimental vaccine that was developed here by the talented scientists at the IIBR. We wish success during these and the latter stages. With G-d’s help, we will have a vaccine made here in Israel. This is a very big thing.”
The clinical trial on human participants with the Brilife vaccine developed by the IIBR will unfold over several months, in three distinct phases.

Harel and another participant at the Hadassah Medical Center in Jerusalem will have received the first vaccine shots on Sunday, after which the first phase will begin with the participation of 80 healthy volunteers (aged 18-55), designated by Sheba and Hadassah (40 in each center).
Each participant will receive an injection (vaccine or placebo), and will be discharged after a few hours of supervision and monitored closely over a three-week period.
Scientists will look for any possible side effects and monitor for antibodies to the virus. The development of antibodies will indicate a response in the patients who received the vaccine.
The second phase will include extensive safety tests with 960 healthy participants over the age of 18. This phase is expected to start in December in several medical centers across the country.
In this phase, scientists will aim to complete safety precautions, determine the effective dosage for the vaccine, and further prove its effectiveness.
Sign up for our free weekly newsletter
Subscribe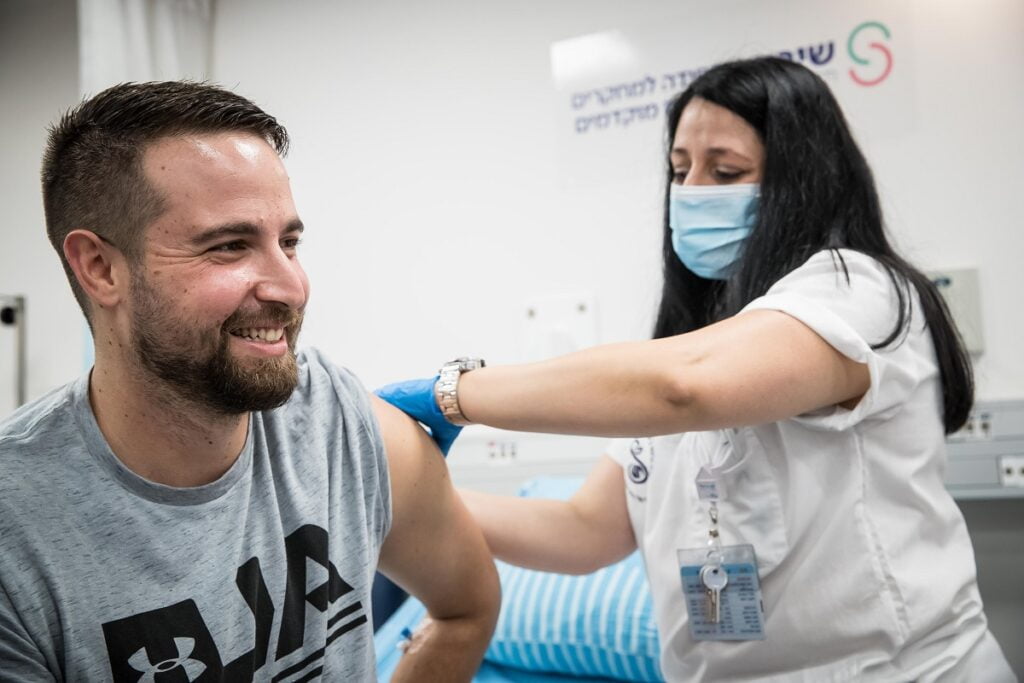
In the third and final stage, up to 30,000 volunteers will take part in the trial for the vaccine.
This stage is subject to the success of the two previous phases and is scheduled to begin in April/May. Should all three phases go well, the vaccine may be approved for mass use, according to the ministry.
Last week, the Defense Ministry said the IIBR has produced more than 25,000 vaccine doses for the first and second phases of the clinical trial and can undertake large-scale production of vaccines – approximately 15 million doses.
SEE ALSO: New Antibody Cocktail May Provide COVID-19 Immunity For Months, Say Israeli Scientists
The Ness Ziona-based research institute has been at work since February, when first tapped by Netanyahu, to develop a vaccine for SARS CoV-2, the virus that causes COVID-19, with several breakthroughs along the way.
In summer, the institute’s scientists said that their vaccine candidate used vesicular stomatitis virus (VSV), an animal virus that does not cause disease in humans, and in which the spike protein was replaced with that of SARS-CoV-2. VSV is also the basis for a separate, effective vaccine against the Ebola virus.

The vaccine, which the scientists called a recombinant VSV-ΔG-spike or rVSV-ΔG-spike, had been tested on a number of animal models, including golden Syrian hamsters, mice, rabbits, and pigs, and was shown to be safe and well-tolerated, and able to bind and neutralize SARS-CoV-2 efficiently.
The vaccine’s commercial name is Brilife, a combination of “Bri” which alludes to the Hebrew word for health, “briut,” “il,” for Israel, and “life.”
Covering all bases
While local vaccine development is ongoing, Israel also has a number of agreements with governments and companies working on separate COVID-19 vaccines including Moderna, a Massachusetts-based firm that was the first to develop an experimental vaccine that went into trial quickly.
Moderna recently wrapped enrollment of some 30,000 participants for its Phase III trial and may begin seeking regulatory approval next month.
Netanyahu said on Sunday that Israel has been in talks with the US, Germany, India, Russia, and Italy about COVID-19 vaccines and promised to work to procure doses -whether locally or from aboard – for everyone.
“With the independent production that has been developed here or through the importing of vaccines from abroad, we will bring enough vaccines for all citizens of Israel. Then we will be able, at long last, with G-d’s help, to be free of the pandemic. I do not think that this will happen immediately but I do tell you that I already see the light at the end of the tunnel – I see vaccines in the State of Israel,” he said.
Netanyahu added that he is looking to set up a non-profit enterprise for the permanent production of vaccines in Israel as a part of national security “just as we do with F-16 squadrons or any other thing that is essential.”
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer




Facebook comments