The government-run Israel Institute for Biological Research (IIBR) has received final approval to launch clinical trials with human participants for its COVID-19 vaccine candidate on November 1, the Israeli Ministry of Defense announced on Sunday.
The Health Ministry and the Helsinki Committee, a medical panel comprised of physicians and advocates that weighs research approval for human experiments, gave the okay for trials to start after “rigorous preparations and R&D,” said the Ministry of Defense.
SEE ALSO: New Antibody Cocktail May Provide COVID-19 Immunity For Months, Say Israeli Scientists
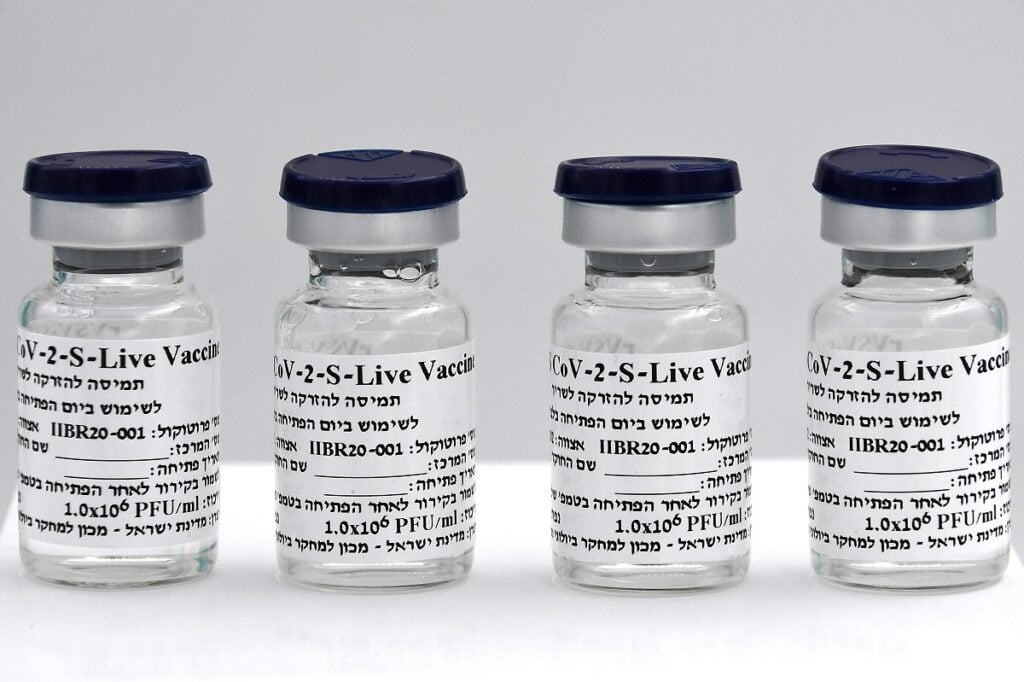
The newly announced commercial name for the Israeli-developed, single-dose vaccine is Brilife, a combination of “Bri” which alludes to the Hebrew word for health, “briut,” “il,” for Israel, and “life.”
“We are now beginning a crucial phase [in the development of the vaccine]: the clinical trials phase,” said Professor Shmuel Shapira, the director of IIBR. “I believe in the abilities of our scientists and I am confident that we can produce a safe and effective vaccine.”
The institute said that, to date, it has produced more than 25,000 vaccine doses for the different phases of the clinical trials and has adapted a device for large-scale production of vaccines – approximately 15 million.
The Ness Ziona-based research institute has been at work since February, when tapped by Prime Minister Benjamin Netanyahu, to develop a vaccine for SARS CoV-2, the virus that causes COVID-19, with several breakthroughs along the way.
In summer, the institute’s scientists said that their vaccine candidate used vesicular stomatitis virus (VSV), an animal virus that does not cause disease in humans, and in which the spike protein was replaced with that of SARS-CoV-2. VSV is also the basis for a separate, effective vaccine against the Ebola virus.
“In this way, the body thinks it has been infected with the real [corona] virus, but actually it’s just a ‘costume,” said Dr. Hadas, a vaccine developer at the IIBR whose full name cannot be revealed, in a video put out by the institute. “So the body develops antibodies against the genetically engineered virus, but in the moment of truth, the body can identify, bind, and neutralize the virus.”
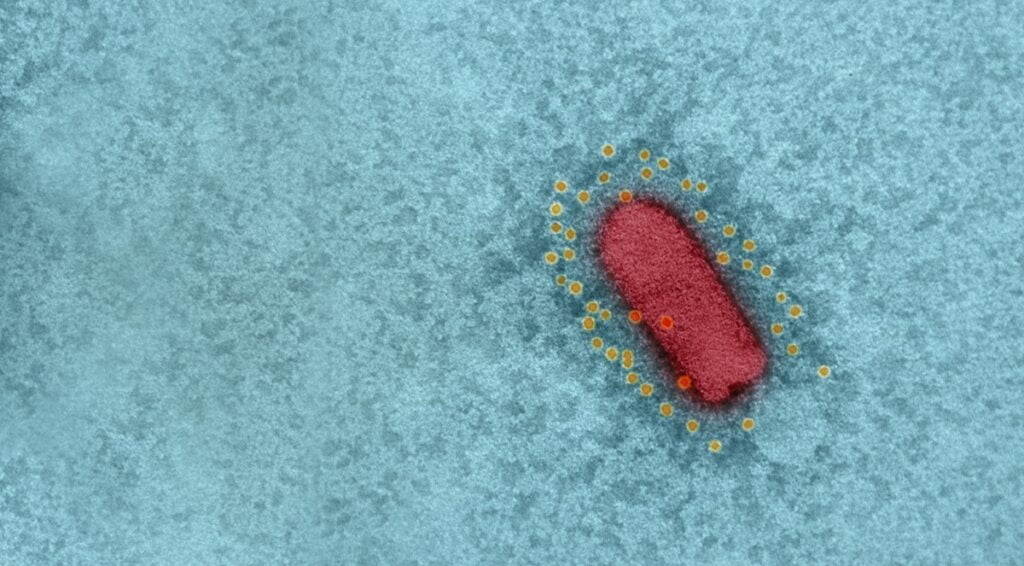
The vaccine, which the scientists called recombinant VSV-ΔG-spike or rVSV-ΔG-spike, had been tested on a number of animal models, including golden Syrian hamsters, mice, rabbits, and pigs, and was shown to be safe and well-tolerated, and able to bind and neutralize SARS-CoV-2 efficiently.
Clinical trial in three phases
The clinical trials on human participants will be conducted over several months, and will include three distinct phases, the Ministry of Defense said Sunday.
In the first phase, safety tests will be conducted with the participation of 80 healthy volunteers (aged 18-55), designated by the Sheba and Hadassah medical centers (40 in each center. The trial will officially begin on Sunday, November 1 with two initial participants.
The vaccine will then be gradually administered to the 80 volunteers. Each participant will receive an injection (vaccine or placebo), and will be discharged after a few hours of supervision and monitored closely over a three-week period.
Scientists will look for any possible side effects and monitor for antibodies to the virus. The development of antibodies will indicate a response in the patients who received the vaccine.
The second phase will include extensive safety tests with 960 healthy participants over the age of 18. This phase is expected to start in December in several medical centers across the country.
Sign up for our free weekly newsletter
SubscribeIn this phase, scientists will aim to complete safety precautions, determine the effective dosage for the vaccine, and further prove its effectiveness.
In the third and final stage, up to 30,000 volunteers will take part in the trial for the vaccine.
This stage is subject to the success of the two previous phases and is scheduled to begin in April/May. Should all three phases go well, the vaccine may be approved for mass use, according to the ministry.
Defense Minister Benny Gantz thanked the institute’s scientists and researchers “who work day and night on this national mission in full cooperation with the Ministry of Health.
SEE ALSO: Cholesterol Drug May Lessen COVID-19 Threat To That Of Common Cold – New Study
“In this complex period, you are the ‘commando unit’ paving the way for the citizens of Israel. You took on a mission of international and historical importance. The defense establishment, the Israeli government, and I will continue to provide you with the support and resources required to produce a safe and effective ‘blue-and-white’ vaccine,” he said.

Netanyahu wished the scientists success at a cabinet meeting earlier in the day and said the government was also working to bring vaccines from abroad to be prepared. Israel has a number of agreements with companies working on separate COVID-19 vaccines including Moderna, a Massachusetts-based firm that was the first to develop an experimental vaccine that went into trial quickly.
Moderna recently wrapped enrollment of some 30,000 participants for its Phase III trial and may begin seeking regulatory approval next month.
Unknown threat
The IIBR is a governmental research center specializing in biology, chemistry and environmental sciences and falls under the jurisdiction of the Prime Minister’s Office.
The institute was first established in 1952 and has done extensive research over the years on disease processes, vaccine planning and development, and detection and identification methods. It employs hundreds of scientists, some of them leaders in their fields in Israel and around the world.
The center said it has been preparing for several years for a scenario with an unknown threat (which it dubbed Unknown X).
“As part of its scientific assessments, the institute purchased and developed advanced platforms, including infrastructure for the rapid identification of epidemic pathogens and tools for the rapid design of effective vaccines in response to outbreaks,” the Defense Ministry said.
Animal models were also put in place to quickly test the safety and efficacy of vaccines and treatments, and new infrastructure were developed for the rapid production of vaccine doses, under stringent regulatory conditions.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer




Facebook comments