Israeli researchers at the Weizmann Institute of Science have devised a breakthrough method for growing mouse embryos outside the womb for longer than ever before — with a nervous system, heart, stomach, and limbs.
The study, published in the Nature journal last week, gives researchers an “unprecedented tool” for understanding how mammalian organs and tissues form, a process that was previously difficult to study because it was hidden inside the mother’s uterus, the university said in an announcement.
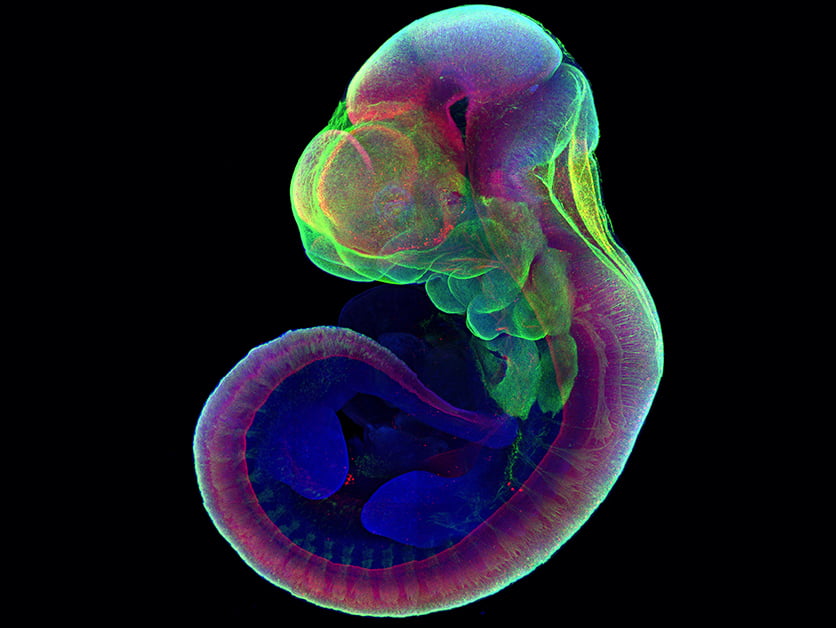
The research was led by Professor Jacob Hanna of the Department of Molecular Genetics and his lab team, which includes Ph.D. student Alejandro Aguilera-Castrejon, Dr. Bernardo Oldak, the late Dr. Rada Massarwa and Dr. Noa Novershtern, and Dr. Itay Maza, a former student of Hanna’s now in the Rambam Health Care Campus of the Technion – Israel Institute of Technology.
The idea of growing early embryos outside the uterus until advanced stages has been around since before the 1930s, but experiments based on these proposals had limited success and the embryos tended to be abnormal, Hanna said in the announcement.
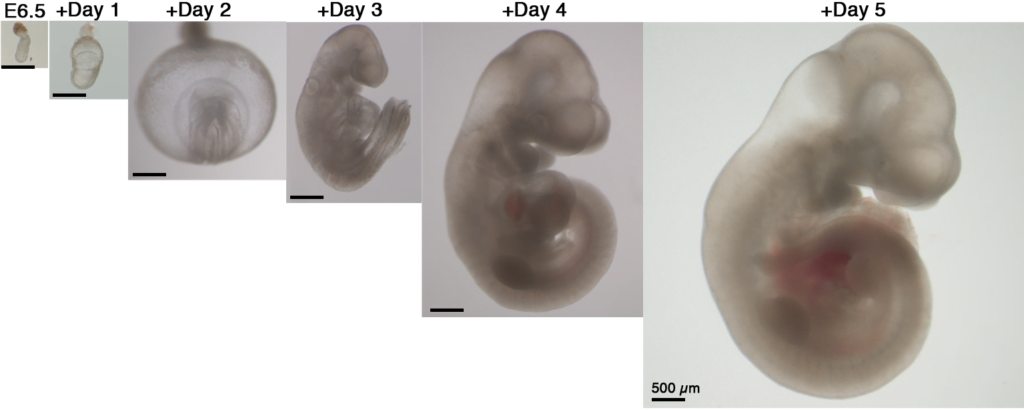
Aguilera-Castrejon tells NoCamels that previous studies were able to grow embryos for 1.5 days maximum, but that their team conceived a method that kept the mouse embryos alive for six days of development.
The Jacob Hanna Lab for Pluripotent Stem Cell Studies and Epigenetic Reprogramming at Weizmann focuses on the way the development program is enacted in embryonic stem cells. Over the course of seven years, Hanna’s team came up with a two-step process in which they were able to grow normally developing mouse embryos outside the uterus for six days, around a third of their 20-day gestation.
Aguilera-Castrejon joined the lab about four-and-a-half years ago and has been the lead on the project ever since.
“We took embryos from the maternal uterus at day five of gestation and found conditions to grow them until day 11. There were six days of normal development of the embryos outside the womb,” he explains. “The interesting part is that precisely during that time (day five to 11), the embryos grow from a ball of cells into an animal with formed hind limbs and all their major organs.”
Day 11 is about halfway through the animals’ 20-day gestation.
Aguilera-Castrejon says the team discovered that the embryos “intrinsically have all the information they need to grow and develop properly.”
“Previously it was thought that it was impossible to grow mammalian embryos in vitro because they needed signals from the mother. Now we show that the embryos can develop without interaction with the maternal uterus,” he says.
The ability to understand how a mammalian embryo can make all of the specific cell types in the body has been one of the fundamental questions in biology, according to Aguilera-Castrejon.
“This technique we have developed can be used to help understand how normal development happens and how it can sometimes go wrong,” he says.
Sign up for our free weekly newsletter
Subscribe
Until now the only way to study the development of tissues and organs was to turn to species like worms, frogs, and flies that do not need a uterus.
“With our system, we can study how organs and tissues are formed in mammals, which can help us to develop ways to recapitulate organ formation in vitro,” he says.”
While the idea of applying this method to a human embryo “is still decades away with the current technology,” Aguilera- Castrejon tells NoCamels the knowledge of this system “will be fundamental for the future creation of organs for replacement therapies in vitro.”
Growing an embryo outside the uterus
To successfully grow embryos outside of the uterus for six days, the team first removed several-day-old mouse embryos right after they would have been implanted. They then isolated the 250 stem cells that made up the embryo, which would normally attach to the uterine wall of the mother. These stem cells were placed on a special growth medium in a laboratory dish and from there grew as they would in a uterus. The team succeeded in duplicating the first stage of embryonic development, where the embryo doubles and triples in size, as it differentiated into three layers.
After two days, the Israeli researchers moved the embryos to rotating glass bottles bathed in nutrients, which kept the mouse embryos alive until day 11 of development. The bottles simulated the blood flow to the placenta and the researchers were careful to control exact balances of oxygen, carbon dioxide, gas pressure, and other elements.
The team conducted careful comparisons with embryos from pregnant mice in the relevant time period, to check whether the developmental processes they observed throughout the two steps were normal. The separation into layers and the organ formation were identical in the two groups.

In subsequent experiments, they inserted genes that labeled the growing organs in fluorescent colors into the embryos. The successful attempt suggested that further experiments with this system involving various genetic and other manipulations should produce reliable results.
“We think you can inject genes or other elements into the cells, alter the conditions or infect the embryo with a virus, and the system we demonstrated will give you results consistent with development inside a mouse uterus,” Professor Hanna said.
SEE ALSO: Israeli Researchers Find Genetic Mutation That May Pave Way For Autism Drugs
“If you give an embryo the right conditions, its genetic code will function like a pre-set line of dominos, arranged to fall one after the other,” he added. “Our aim was to recreate those conditions, and now we can watch, in real-time, as each domino hits the next one in line.”
The method will lower the cost and speed up the process of research in the field of developmental biology, as well as reducing the need for lab animals, among other things, according to Prof. Hanna.
The next step in Hanna’s lab will be to see if they can skip the step of removing embryos from pregnant mice. The team intends to try to create artificial embryos made from stem cells for use in this research. They hope to put their new method to work to answer such questions as to why so many pregnancies fail to implant, why the window for implantation is so short, how stem cells gradually lose their “stemness” as differentiation progresses and what conditions in gestation may later lead to developmental disorders.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer



Facebook comments