In February 2012, GE’s $6 billion Healthymagination initiative adopted its first Israeli project: a breakthrough solution for colorectal cancer screening.
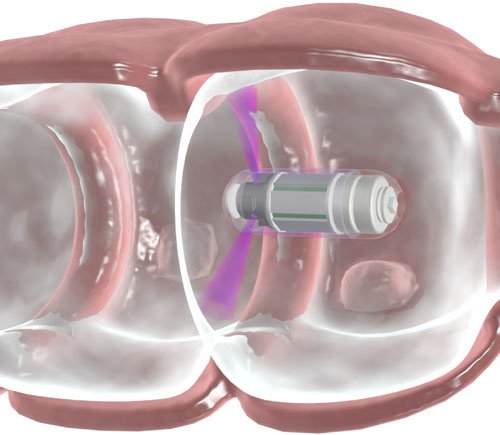
“One of the reasons so many people die from colon cancer, despite it being relatively easy to cure, is because they avoid the uncomfortable and invasive colonoscopy procedure,” says Guy Neev, CEO of Check-Cap, which has designed an ingestible capsule that takes 360-degree X-ray snapshots of the colon as it travels through the gastrointestinal tract.
“The capsule goes in and out of the body within days, and is designed to detect clinically significant polyps within the same range of accuracy as a standard colonoscopy,” he explains.
Related Stories:
- Biodegradable Balloons Isolate Tumors To Reduce Radiation Risks
- New ‘Seek And Destroy’ Method To Fight Cancer
Check-Cap, a seven-year-old company based in the Druze town of Isfiya on Mount Carmel, was founded in early 2005 by its Chief Technology Officer Dr. Yoav Kimchy, a physics specialist and technical project leader.
He brought together a 30-strong team of experts in fields as diverse as electronics, physics mathematics, mechanics, software engineering, chemistry, physiology and medicine – mostly graduates of the Technion-Israel Institute of Technology in nearby Haifa – to produce a device that could forever revolutionize colon cancer detection, prevention and treatment. “The challenge was to produce something less invasive than current procedures,” Neev emphasizes.
The concept of using technology in a pill for visualizing and detecting disorders of the GI tract was pioneered by Israel’s Given Imaging. Check-Cap uses a different approach that’s even more patient-friendly, says Neev.
“While the currently available capsule endoscopies are based on cameras and therefore require aggressive bowel cleansing, the Check-Cap imaging capsule is the first device designed to work in the colon with virtually no bowel preparation. That is possible because it employs low-energy X-ray which can see through the colon content.”
The patient doesn’t even have to be in a hospital or doctor’s office during the procedure. “They can go about their daily routines without having to alter their activities,” says Neev.
Minimal radiation
The system, which has already been proven in animals, includes a capsule, a receiver, proprietary imaging software and an associated database for web-based access and analysis.
Check-Cap’s capsule features miniature cadmium zinc telluride (CZT) diagnostic imaging sensors. The tiny X-ray radar device creates a 3D reconstructed image of the colon with minimal exposure to radiation – far less than a CT scan or mammography, for example.
The capsule transmits data to a wrist-worn device, where it is stored for a physician’s analysis. If the 3D images reveal polyps, a therapeutic colonoscopy will be prescribed as needed.
Sign up for our free weekly newsletter
Subscribe“The product is ready to be tested,” says Neev. “We anticipate asking for European marketing approval by the end of next year, and in the US probably 18 months later.”
The aim is to launch the imaging capsule in the European Union in late 2013, subject to CE Mark regulatory approval. Discussions with the US Food and Drug Administration (FDA) about clinical activities to support approval to market the product in the United States are well underway.
Enormous potential market
Colorectal cancer is the third most common cancer in men and the second in women, killing 608,000 people a year worldwide – eight percent of all cancer deaths – according to the International Agency for Research on Cancer. It is the fourth leading cause of cancer death in the world.
This means that the fledgling Israeli company should soon have a foothold in a market potentially worth $25 billion. “The potential is enormous: About 350 million people in the Western world alone,” says Neev.
“Our goal is to reduce patient mortality by facilitating dramatically increased patient adherence with the physicians’ screening recommendations, allowing earlier detection and treatment,” he says. “Everyone over 50 owes themselves a colon cancer screening – after all, early detection saves lives.”
First collaboration in Israel
This is the first investment in Israel for the GE Healthymagination fund – initiated to help deliver better healthcare to more people at a lower cost. General Electric’s equity investment fund focuses on identifying and partnering with promising healthcare technology companies worldwide.
“We are pleased to have GE as a new investor and collaborator,” say Neev. “GE’s investment is an acknowledgement of the patients’ need we are addressing as well as the clinical promise of our technology. Colon cancer is the most deadly, yet preventable cancer. GE’s experience in the imaging space will be a significant contribution to our efforts as we progress in our clinical and regulatory program towards commercialization.”
Several US and Israeli venture capital and private equity firms, plus some European firms, also support Check-Cap. The company’s shareholders include Pontifax Venture Partners, Biomedix, DoCor, the Emigrant savings bank, Counterpoint, Jacobs Investment Company, BXR Partners and private investors.
Photos courtesy of Check-Cap
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer




Facebook comments