Israel’s COVID-19 vaccine developer, the government-run bio-defense firm Israel Institute for Biological Research (IIBR), has signed an agreement to complete clinical trials and commercialize its coronavirus jab BriLife with NRx Pharmaceuticals, a US clinical-stage pharmaceutical company traded on the Nasdaq
The deal was announced Monday by the Israeli Ministry of Defense and grants NRx Pharmaceuticals exclusive worldwide license to further develop, manufacture, and market BriLife. The vaccine’s name is a combination of “Bri” which alludes to the Hebrew word for health, “briut,” “il” for Israel, and “life.”
SEE ALSO: Israeli COVID-19 Vaccine Developer Said Aiming For Single Dose, May Redo Trials
The Ness Ziona-based IIBR launched the second phase of its clinical trial in December with 1,000 volunteers aged 18 and over after the first phase, which began November 1 with 80 volunteers, was completed successfully with no significant side effects. A third phase with up to 30,000 volunteers in Israel and/or abroad was supposed to begin this past spring amid reports that the institute sought to develop a one-dose version of the jab while also eyeing a redo of the clinical trials, possibly in Argentina. This was due to Israel’s rapid vaccination rate, with over 70 percent of Israelis aged 16 and over having been inoculated.
The Defense Ministry told NoCamels in May that the clinical trial of the vaccine developed at the biological institute was progressing as planned, and that “there was never a need to repeat the first phase.”

This week, NRx Pharmaceuticals said it has arranged for rapid Phase IIb and Phase III testing of BriLife in Georgia and Ukraine — in addition to Israel — with support from the respective governments and health ministries, and in cooperation with US-based company Cromos LLC, a regional contract research organization (CRO) specializing in clinical trials.
According to the agreement, NRx will conduct clinical trials on tens of thousands of volunteers in Georgia and Ukraine and IIBR will accompany the process and continue to share knowledge and provide scientific assistance to complete the trials.
Israel’s BriLife vaccine is a self-propagating, live-virus vaccine that uses vesicular stomatitis virus (VSV), an animal virus that does not cause disease in humans, and in which the spike protein was replaced with that of SARS-CoV-2, the virus that causes COVID-19. VSV is also the basis for a separate, effective vaccine against the Ebola virus.
The vaccine is based on a previous, FDA-approved vaccine platform that was further optimized by IIBR, NRx said. It is different than the Pfizer and Moderna mRNA vaccines, a type of jab that teaches our cells how to make a protein that triggers an immune response, rather than introducing a weakened or inactivated virus which has been the traditional vaccine approach.
Israel has used the Pfizer jab in its ongoing, successful vaccination program.
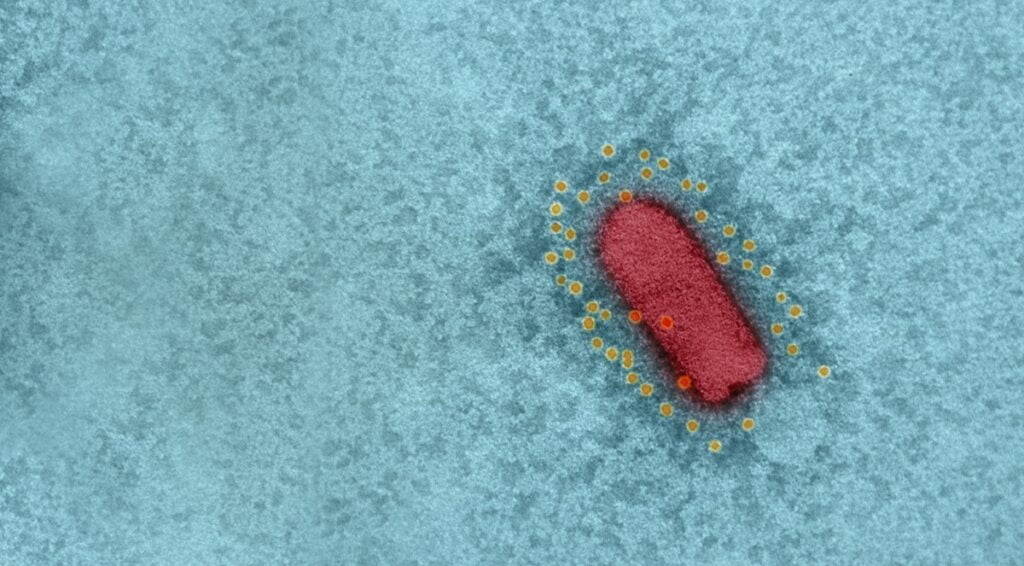
The BriLife vaccine, which the IIBR scientists called a recombinant VSV-ΔG-spike or rVSV-ΔG-spike, had previously been tested on a number of animal models, including golden Syrian hamsters, mice, rabbits, and pigs, and was shown to be safe and well-tolerated, and able to bind and neutralize SARS-CoV-2 efficiently.
Sign up for our free weekly newsletter
SubscribeThe full results for the BriLife vaccine have not been made public, so its efficacy against COVID-19 is lesser widely known than established vaccines like Pfizer’s and Moderna’s.
But NRx said it anticipates rapid and affordable industrial scaleup and manufacturing for BriLife which will initially be delivered by traditional injection.
The initiative will be led by NRx Chairman and CEO Professor Jonathan Javitt together with NRx Director Chaim Hurvitz, a former director of Teva Pharmaceuticals who chairs CH Health, a Tel Aviv-based private equity group.

“We at NRx are honored to have been selected by the Government of Israel to carry forward this life-saving mission,” said Hurvitz in a company statement. “As the first generation COVID vaccines are increasingly challenged by rapid mutation of the coronavirus, we aim to develop a vaccine that can rapidly scale at low cost to serve the needs of both the developed and the developing world.”
Indeed, scientists and researchers across the world are closely watching surges of infections with the COVID-19 Delta variant (previously known as the India variant and B.1.617.2), a more easily transmitted strain caused by mutations on the spike protein. It is the dominant strain in the US and the UK, and Israel, which recently reimposed certain restrictions such as mask-wearing, as cases have shot upward over the past few weeks though severe cases remain relatively low.
The Israeli Health Ministry announced last week that the Delta variant was found to be more resistant to the vaccine. According to a recent analysis by the ministry, the Pfizer vaccine was considered 64 percent effective at preventing symptomatic and asymptomatic disease and also 93 percent effective at preventing hospitalization after infection, and death.
“In its first year, the COVID pandemic has killed four million people worldwide, constituting the worst public health catastrophe since the 1918 Influenza epidemic,” said Javitt, who has been involved in the fight against coronaviruses since the 2002 SARS epidemic.
“As the virus mutates and challenges the immunity we have built through first-generation vaccines, we are excited to expand our focus on COVID by including a vaccine platform that has [the] potential to scale at high speed and low cost,” he added in the statement.
Israeli Defense Minister Benny Gantz praised the new agreement and said it was “excellent news for Israel’s citizens” and “highlights the unprecedented achievement of the Israel Institute for Biological Research.”
“I anticipate that with this agreement, we will be able to complete the development of the vaccine and enable Israel to produce vaccines independently, because as we have seen recently – the coronavirus is not going anywhere. At the same time, the IIBR and the entire defense establishment will continue to take part in the national effort to counter the effects of this pandemic,” added Gantz.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer



Facebook comments