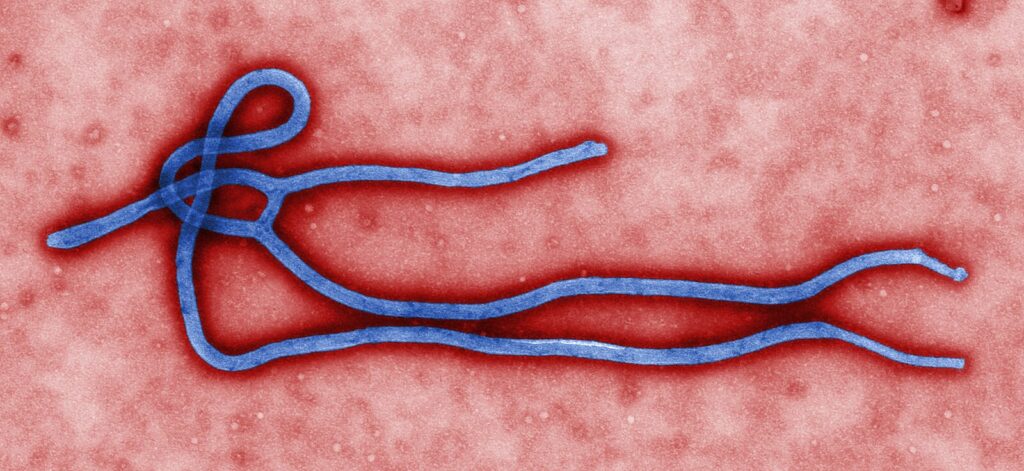
The US government is funding the development of an effective treatment created by Israeli firm RedHill Biopharma for the rare and deadly Ebola virus (EBOV).
The funding comes via the Biomedical Advanced Research and Development Authority (BARDA), part of the US Department of Health and Human Services’ Administration for Strategic Preparedness and Response (ASPR).
A study last year carried out by RedHill found that twice-daily oral doses of its opaganib medication boosted survival from about six days to 11 days in mice infected with Ebola.
Opaganib is a host-directed therapy, meaning rather than destroying the pathogen directly, it makes its local environment less favorable to grow and live in.
The drug is also in development as a treatment for multiple cancers, COVID-19 and viral and inflammatory diseases.
“EBOV is deadly, killing, on average, half of all those who contract it. This year marks 10 years since the West Africa Ebola epidemic in which 11,000 people died, and yet there are still no host-directed, small molecule therapies approved to provide effective and usable treatment strategies,” said RedHill Chief Business Officer Guy Goldberg.
“Currently only Inmazeb, a combination of three monoclonal antibodies, and Ebanga, a single monoclonal antibody, are FDA-approved to treat EBOV, as such there is an urgent medical need for additional effective and easy to distribute and administer EBOV therapies,” he said.
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments