A hunch by a respected Israeli biochemist about a molecule found in papaya has opened the door to revolutionize the treatment for metabolic and respiratory disorders, including cystic fibrosis (CF) in children.
Inspired by the hunch, Israeli biotech startup ODE Pharma was founded with the mission of developing safe and cost-effective treatments for children suffering from the genetic disease.
CF is caused by a defective gene that makes the body produce thick and sticky mucus in the lungs and other organs, which leads to a variety of serious lifelong issues, including chronic respiratory problems and recurrent lung infections.
There is no cure for the disease, and although a range of treatments have been developed to ease its symptoms, they can be expensive and are limited in scope.

But ODE believes its new therapy can make a difference to young people suffering from the disease and other respiratory disorders, by healing lung cells that have been damaged.
“We want people to go from uncomfortable to comfortable,” ODE Pharma CEO Assaf Bivas tells NoCamels.
The company focused on cystic fibrosis in children because they believed their molecule could have a real impact on a disease that had hitherto received little attention and was very costly for parents – both in terms of managing CF and the toll it took on the families.
“Beyond focusing solely on profits”, Bivas says, “our mission is to bring happiness and health to individuals of all ages affected by respiratory and pulmonary conditions, such as cystic fibrosis.”
This is also the overall approach of the Pollen Innovation Group, the investment group backing ODE Pharma that was co-founded by Bivas, Asaf Ofer, Ofer Shapira, and Dave Wolf.
Asaf Ofer is also the CEO of Kenaf Ventures, a cleantech startup based in southern Israel that creates a versatile biomaterial from the easily cultivated Kenaf plant.
ODE is utilizing its novel molecule ODE-001, to enhance cellular resilience against inflammation and other forms of deterioration.
The late Prof. Shmuel Ben-Sasson, upon discovering the molecule, partnered with the Pollen Innovation Group, joined by renowned chemist and Nobel laureate Prof. Aaron Ciechanover.
Ben-Sasson’s groundbreaking discovery of the naturally occurring molecule within papaya fruit revealed its potential to revitalize lung tissue function and enhance energy production. This innovative compound, subsequently named ODE-001, offered a promising avenue for symptomatic relief.
Ben-Sasson’s distinguished scientific credentials lent credibility to his intuition regarding the molecule’s therapeutic potential.
Sign up for our free weekly newsletter
SubscribeThe Pollen Group swiftly acquired the intellectual property rights to the molecule, completing the IP transfer in just one month.

Then they first tested the molecule in a lab, inserting it into the epithelial tissue (a thin protective layer covering all the surfaces of the body) of healthy cells found in both the lungs and the intestine.
Bivas says the team was thrilled to learn that ODE-001 did indeed extend the life of the healthy cells by almost 50 percent. And when they moved to test inflamed cells, the team found that it was just as – if not more – effective.
“We found that [the molecule] is a very, very strong anti-inflammatory agent that… rehabilitates the cell itself,” says Bivas. “We had something.”
A clinical trial conducted in association with one of Israel’s four health care providers further yielded “very good and promising results,” he says.
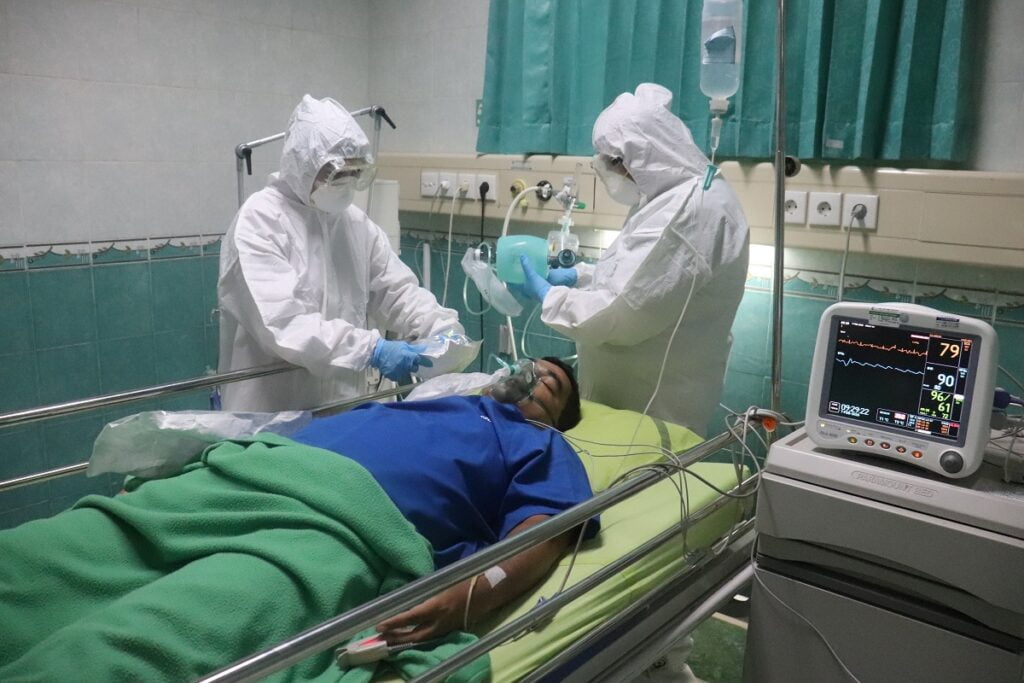
During the height of the COVID-19 pandemic, ODE conducted a clinical trial involving 13 patients in an Israeli intensive care unit.
“These patients received ODE-001. Remarkably, all 13 patients were discharged from the hospital within five days of treatment, significantly outperforming the control group of 59 patients who did not receive ODE-001,” Bivas says.
And while the control group experienced a 24 percent mortality rate, he adds, no patient in the ODE-001 group died.
Newly emerged from stealth, ODE Pharma has so far raised $1.5 million in funding and is in the process of raising another $5 million.
It plans to start clinical trials for ODE-001 as a treatment for cystic fibrosis in children next year, with the aim of seeking orphan drug designation from the US Food and Drug Administration, a classification that applies to the development of novel treatments for rare diseases in the US.
And with signs that ODE-001 helps mitigate damage from COVID and helps cells to fight inflammation, ODE has plans to release the molecule as a supplement for respiratory issues in the next year.
The company is working with Catalent, a multinational biotech manufacturer based in New Jersey with more than 50 factories, to mass produce the supplement.
“With its exceptional safety, affordability, and potential for large-scale production, this compound is poised to make a significant impact,” Bivas says.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer



Facebook comments