Israeli researchers have devised a new way of treating cancerous cells in bone marrow – for the first time taking the therapy directly to the cancer inside the soft, fatty tissue contained within our bones.
This revolutionary method involves surrounding RNA, a molecule containing instructions for cellular behavior, with targeted lipid nanoparticles that send the treatment straight into the cancerous cells in bone marrow, known as myeloma.
Once inside those cells, the RNA molecules act to inhibit the growth of a certain protein, thereby effectively killing the cancer cells by preventing them from dividing.
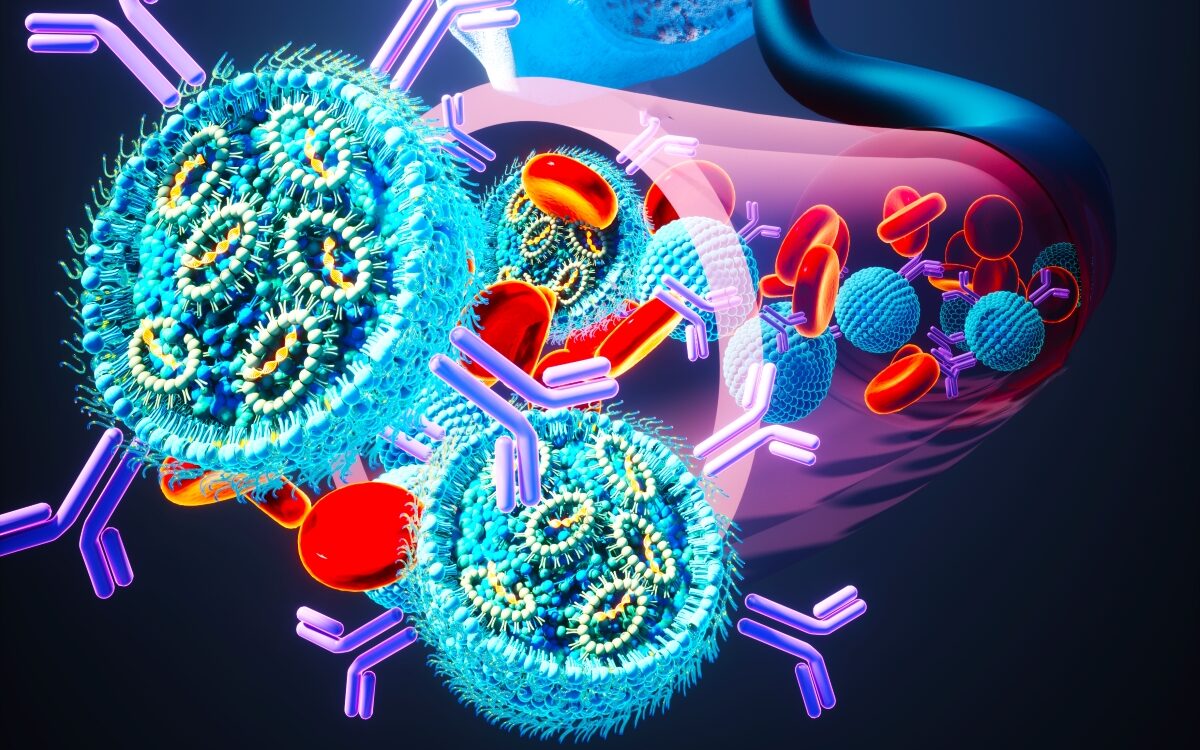
This is the same method of drug delivery as used in the COVID vaccines developed by pharma giants Pfizer-BioNTech and Moderna. In the case of the vaccines, the RNA recreated the spike proteins on the outside of the coronavirus in order to teach the immune system to produce antibodies to fight them.
The development is the work of scientists at Tel Aviv University, led by Prof. Dan Peer, the head of the Nanomedicine Laboratory at the Shmunis School of Biomedicine and Cancer Research and TAU’s vice president of R&D, with PhD student Dana Tarab-Ravski.
The researchers collaborated with counterparts at Rabin Medical Center in Petah Tikva, one of the largest medical institutions in Israel. The results were published in the Advanced Science journal.
From COVID To Cancer
Peer tells NoCamels that the lipid nanoparticles act as a “GPS system” for the drugs to target myeloma.
“We take exactly the same concept as the mRNA [a form of RNA] vaccines,” he says. “On the surface of those particles, we put the GPS system that when you inject it into the bloodstream directs it into the bone marrow. And within the bone marrow, we are going after myeloma cells.”
Billions of new blood cells are created in our bone marrow every day, including red blood cells, platelets and white blood cells. Myeloma is cancer of plasma cells – the type of white blood cells that make antibodies to fight infection.
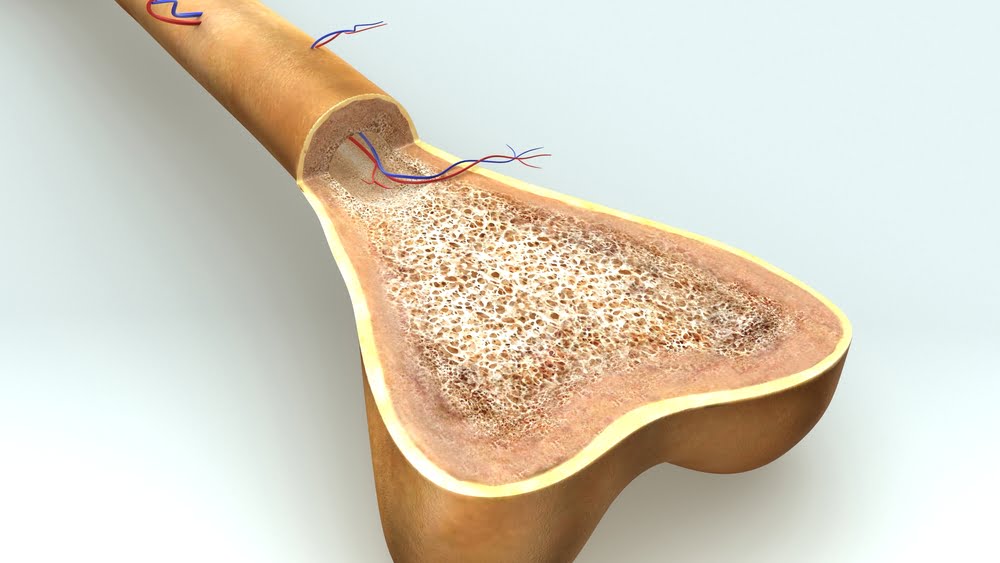
Myelomas in bone marrow overwhelm the other cells there, and because they are closely connected to the blood that circulates throughout the body, can easily spread.
“People with multiple myeloma suffer from severe pain in their bones, as well as anemia, kidney failure, and a weakened immune system,” said Tarab-Ravski.
According to Peer, the new treatment destroyed 90 percent of myeloma cells in the lab and 60 percent of the cells in human tissue.
“The efficiency by which our system goes to the bone marrow, specifically to myeloma cells is very high. And before that, without this kind of a smart GPS system, maybe one percent would go to the bone marrow,” he says.
Ordinarily, Peer explains, when particles are injected systemically into the bloodstream, they end up in the liver, whose job is to clean the body’s blood.
Sign up for our free weekly newsletter
Subscribe“What we wanted was to endow those lipid nanoparticles – with their RNA drug inside – with the ability to target the myeloma cells inside the bone marrow. This is the whole new thing,” Peer says.
The targeted delivery system works by equipping the lipid nanoparticles with “a very selective marker” for the myeloma cells in the bone marrow, he explains.
This involves engineering antibodies to place on the surface of the lipid nanoparticles that will attract them to the unique receptors on the surface of the myeloma cells. While healthy cells in the bone marrow also have these receptors, they are thousands of times more numerous on the myeloma cells.
As such, the nanoparticles are drawn to the cancer cells and not the healthy cells around them.
“It’s very simple mathematics,” Peer says.
Future Potential
The premise of the treatment was already being studied when the coronavirus pandemic hit in early 2020, but the rush for a COVID vaccine boosted the work “dramatically,” according to Peer.
“A lot of money was pushed into the development,” he says. “People saw the engineering challenge here, to put together mRNA and lipids in a controlled manner.”
In fact, he says, “COVID teaches us that it’s doable.”
The therapy is still in pre-clinical trials and for now is planned exclusively as a blood cancer treatment, but Peer says that it could theoretically be used to treat forms of metastatic cancers that spread through the bloodstream.
He warns, however, that penetrating solid tumors that appear in the organs is extremely difficult. Bone marrow is soft tissue, which makes it easier for the drug to reach the cancerous cells.
Peer also says that the treatment could one day be used to create vaccines for cancers.
“Unfortunately,” he says, “we are still not there.”
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer



Facebook comments