A 14-year old boy dying from diabetes in a Toronto hospital became the first person in the world to receive an insulin injection in 1922. Within 24 hours, his high blood glucose levels dropped to near-normal levels and he lived to tell the tale.
For the next 60 years, insulin derived from pigs and cattle was used to treat countless millions of diabetics.
But it was imperfect, complex and expensive to produce, and it caused allergic reactions in many patients. Then, in the early 1980s came another breakthrough – synthetic human insulin.
Today insulin, and a plethora of other products like alcohol, antibiotics, vitamins, and citric and lactic acid, are made through fermentation – chemical processes caused by organisms like bacteria, yeasts, and fungi.
But they are still complicated processes, and manufacturers cannot scale up production to meet the demand for all of the food, pharmaceutical, and cleaning products that we use daily.

Israeli startup Enzymit has simplified things. CEO Gideon Lapidoth says his company’s computer algorithms can design “new to nature” enzymes to order.
“Enzymes are the basic elements you need for life,” he says. “Right now, there’s an enzyme in your eye that’s converting light energy into chemical energy, and that’s how your brain sees the world. And when plants photosynthesize, an enzyme takes light energy and turns it into sugar.”
“So really, the possibilities of what you can do with the right proteins are endless. There’s nothing you can’t do with the right protein.
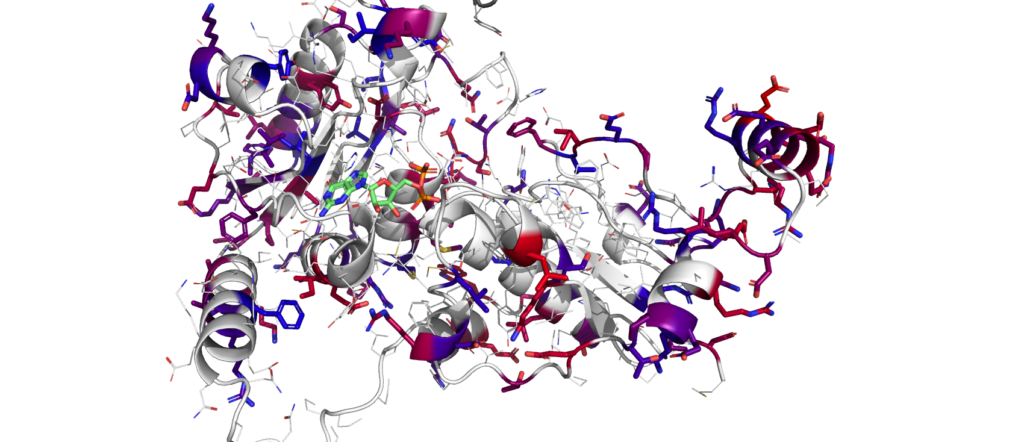
“What we’re saying is, we don’t need the entire bacteria. That’s just a waste of space, a waste of energy, and a waste of resources. All we need are specific enzymes, which are just the molecular machines that actually do the chemistry.”
Enzymes are the proteins that create a chemical reaction. Enzymit tells its algorithm a specific protein sequence that results in an enzymatic process, and inputs the traits it desires – like being extremely heat resistant. The algorithm then generates a new sequence that, when created industrially, will preserve all the functionalities of the original protein.
The algorithm generates enzyme designs on computers and translates it into DNA code. Enzymit works with several companies around the world that literally print the DNA to be used in industrial processes. Beyond that, the startup also employs its algorithms in its own sophisticated machine to create enzymes.

“It’s almost like ordering something from Amazon. You go on the website, type in your sequence or design, they send you DNA, you put the DNA into bacteria, and the bacteria produces your protein,” explains Lapidoth.
Sign up for our free weekly newsletter
SubscribeNumerous companies have already used Enzymit. One is a producer of hyaluronic acid, which is used in cosmetics to reduce scarring and wrinkles, and help wounds heal faster.
The substance is traditionally extracted from rooster combs in butcheries – which is expensive to produce. Enzymit was able to create it with its computer program.

Another project it’s working on is developing enzymes to produce prebiotics (food for healthy bacteria) that are normally found in breast milk.
Many infant formula companies want to introduce these prebiotics into their products, but cannot as they are currently only produced through fermentation and are expensive to manufacture.
“Infant formula in the next few years will not just be a replacement, but a one-to-one simulacrum (fake version of something real) of human milk,” says Lapidoth.

Enzymit is now developing enzymes that will literally be able to break down plastics in a matter of hours, as part of a consortium headed by the Israeli Innovation Authority.
“We know we can do pretty much everything with enzymes. It sounds like I’m exaggerating, but I’m not. We just didn’t have the right enzymes until now.”
Lapidoth says there was never a need for Mother Nature to evolve enzymes that can degrade plastic, for example, because materials made with such long carbon chains aren’t natural.

“Nature is very, very efficient. It only builds what is absolutely needed. But the problem is that we don’t have the time to wait for organisms to develop these enzymes naturally.”
And until now, researchers have gone into various ecosystems to try and find proteins to fit what they need.
“This is an extremely inefficient expedition. A much more desirable way is to just engineer it. Describe what you need, and I’ll build it for you.”
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer




Facebook comments