Brand-new treatment stops tumor cells from ‘hiding’ and is being tested in the US
Cancer therapies have improved greatly since chemotherapy was first used to treat the disease in the 1930s. The newer immuno-oncology treatments – which use substances made by the body’s own immune system to combat cancer – don’t carry the same toxic side-effects that chemo does.
The problem is that immuno-oncology treatments don’t work on certain cancers, like lung, ovarian, and breast cancer, because these cancers produce high amounts of a specific protein known as the poliovirus receptor (PVR), which prevent immuno-oncology treatments from being effective.

But a brand-new drug that was developed by an Israeli company is being tested on cancer patients right now – and it may open the door for immuno-oncology to treat millions of people who were so far ineligible.
“What we’re trying to solve is the issue of lack of response for the majority of patients to the currently approved immuno-oncology therapies,” says Fabian Tenenbaum, CEO of Nectin Therapeutics, the company behind the novel treatment. “Only about 20 percent of the patients that benefit from those therapies see a durable response that stays with them over time.”
In normal tissues PVR is barely produced, but when produced in high concentrations, it prevents the immune system – and immuno-oncology treatments – from killing cancer cells.
“The fundamental discovery behind our treatment is the role that PVR, a common receptor on cancer cells, plays in avoiding our immune system,” he tells NoCamels. “It essentially sends the immune system a ‘do not eat me’ sign.”
One of the most common types of immuno-oncology therapies are immune checkpoint inhibitors. When our immune system attacks invaders like bacteria and viruses, it uses a system of ‘brakes’ called checkpoints to stop it from attacking healthy cells too.
But cancer cells can sometimes use certain proteins – like PD-1 and PD-L1 – to hide from the immune system.
Immune checkpoint inhibitors ‘release the brakes’ on our immune system to kill cancer cells by blocking such proteins. The problem is that when tumors have high levels of PVR, they surpass these drugs and tell immune cells not to attack the cancerous cells.

Nectin’s treatment – called NTX1088 – blocks the PVR so that the PD-1 and PD-L1 therapies can get the job done. “By blocking PVR, we can see in the animal models that we can turn on a much more significant and efficient killing of the tumor cells by immune cells, and eradication of the tumor.”
The company was established in 2017 after Pini Tsukerman, Chief Scientific Officer, discovered the way this protein affected the immune system during his research at the Hebrew University of Jerusalem – along with Prof Ofer Mandelboim, who is on the Scientific Advisory Board of the company, and fellow researchers from the University of Rijeka in Croatia.
“They essentially figured out the role that this family of proteins and receptors play in the interaction between the immune system and the tumor cell,” says Tenenbaum.
Sign up for our free weekly newsletter
Subscribe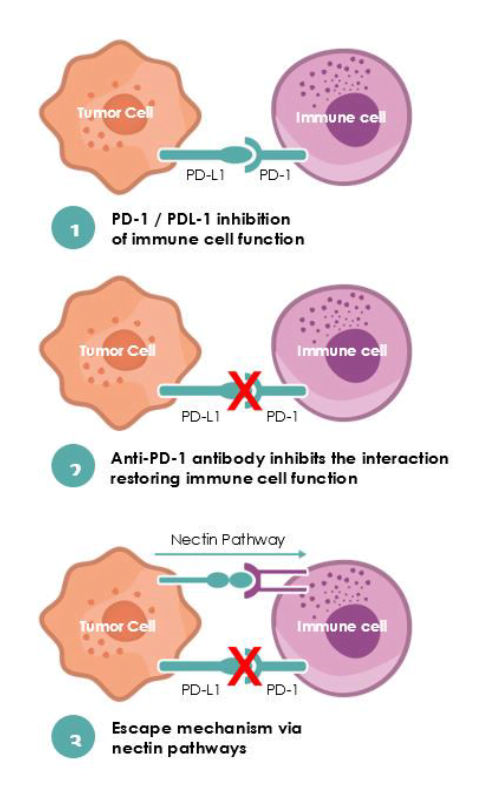
One of Nectin’s main goals is to greatly reduce the need for chemotherapy.
“For many patients it has had an enormous impact both in terms of being really well tolerated, and delivering really potent results, essentially enabling your immune system to fight cancer the way it’s designed to do.”
Tenenbaum does caution that in cases where cancer is advanced, chemotherapy is generally still the only option.
The Jerusalem-based company recently gave its first patient a dose of its novel treatment as part of a clinical trial at the University of Texas MD Anderson Cancer Center, and will include up to 90 patients with locally advanced and metastatic solid tumor, or cancer cells that have broken away from their original tumor and formed a new one in other organs.
“The study starts by dose escalation, where we slowly increase them until we get to what we believe to be the efficacious doses,” says Tenenbaum. Half of the patients will be evaluated with only Nectin’s treatment, while the other will be treated with both immune checkpoint inhibitors, and the company’s drug.
He’s hoping to glean critical information from the trial – like how PVR levels vary in different kinds of tumors, and how much it prevents patients from seeing results when using immune checkpoint inhibitors.
“If PVR plays the role that we believe it does, then it could be affecting a very large number of patients – but it’s hard to say exactly what percentage.”
Next year, the clinical trial will test Nectin’s drug in combination with the PD-1 and PD-L1 therapies.
“We can potentially help a very, very large number of patients, because you’re dealing with some of the most common cancers, including lung and colorectal cancer.”
“The goal is to deliver the optimal benefit to the patients. If you look at melanoma, for example, it is one of the best examples where immuno-oncology has played a very successful role in both delivering survival rates that were unheard of a few years ago, as well as reducing the need for chemotherapy.”
Nectin Therapeutics has around 10 employees, and has raised over $30 million from investors including aMoon Fund, Peregrine Ventures and Integra Holdings.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer




Facebook comments