The COVID-19 pandemic exacerbated an already problematic medical crisis: the shortage of blood in medical settings.
With hospitals at capacity and prospective blood donors quarantined at home these past few years, blood donations fell when they were needed most. As everyday medical procedures took a backseat to COVID-19 patients, a backlog of treatments ensued.
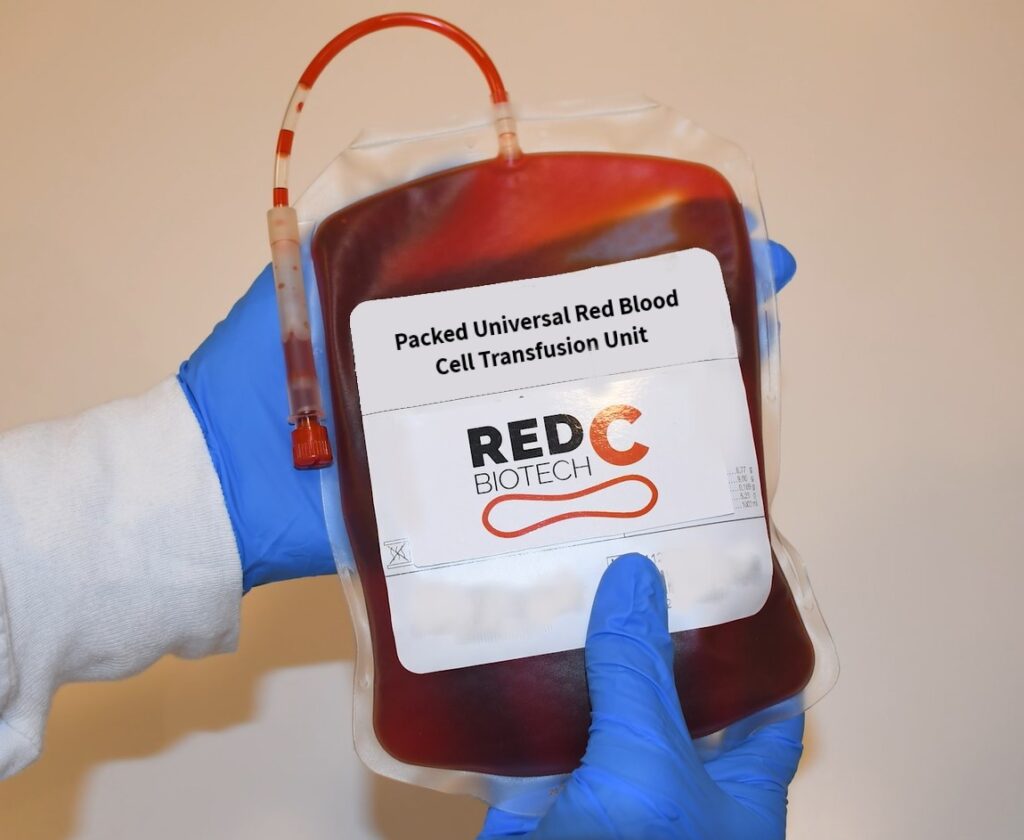
Israeli company RedC Biotech is looking to alleviate blood shortages by developing universal red blood cells in a lab that can then be employed for patients without the need for a donor.
Their lab-produced universal blood cells follow an exact scientific process. First, the scientists create a “master cell bank” by taking stem cells from a donor with universal O negative blood. The stem cells are then cultivated to a very high concentration where they undergo differentiation (the process through which stem cells turn into their target cells), and propagated (multiplied).
RedC Founder and CEO Ari Gargir explains that this process is quite similar to the way that cultivated, or lab-grown, meat is created. “Cultivated meat companies take a stem cell from a cow and they turn it into the components of a steak- muscle, connective tissue, and fat, and compose the steak. We’re taking a human, universal donor stem cell and turning it into a red blood cell.”

“There’s estimated to be at least 100 million blood units that are in shortage around the world,” Gargir tells NoCamels in a Zoom interview. “But this large, problematic shortage does not influence all of the countries around the globe in the same way.”
Gargir became fascinated with this problem in 1990 when he was injured in a paragliding accident in the Golan Heights and needed a blood transfusion.
In order for a country to have a healthy supply of blood transfusions, around three percent of the population needs to be donors, Gargir explains. In this West, this is more or less the case, yet other countries only have around one percent donating; this leaves some countries operating at a major blood deficit.
“Around the world there are about 120 million blood transfusions that are donated every year,” Gargir says. “Most of them are in high income regions. But the problem is mostly in low income regions. Specifically Sub-Saharan Africa where there’s a lot of sickle cell anemia, accidents, and problems with childbirth. Millions of people die every year because of insufficient and inadequate blood transfusions.”
Additionally, bad weather, national holidays, and diseases contribute to donation shortages. In the case of COVID-19, hospitals were forced to cancel non-life threatening operations due to critically low blood supplies.
RedC Biotech will reduce this imbalance through standardized mass production and scaling, making it easier and cheaper to produce and transport blood on a global scale.
Sign up for our free weekly newsletter
Subscribe“The most important part is that the production will be in a factory- in a production facility- and because of that you can produce according to demand and according to a forecast so you don’t fall into shortages,” Gargir says. “You know that this season you’ll need more blood, this one you’ll need less, and you’re able to plan your needs.”
The company recently received international acclaim when they took first place in the inaugural “Aviram Awards- Tech for Humanity” event, a live stage pitch competition hosted by the Aviram Family Foundation and Forbes, which took place in Dubai in March. The competition awarded the most promising startups in the Middle East and North Africa that combine great business with a strong impact on humanity. Gargir and RedC Biotech took home a grand prize of $500,000 and the chance to be mentored by Israeli entrepreneur Ziv Aviram, who co-founded Mobileye and OrCam.
Gargir tells NoCamels that the prize will help in “the exposure and recognition that helps in creating dialogues with potential investors and collaboration.”
National treasure to pharmaceutical commodity
RedC Biotech’s goal also aims to reduce two essential components of blood transfusions that make it difficult to keep up with demand: cost and infrastructure needs.
“In Western Countries, the cost of a blood transfusion is up to $200 per person,” Gargir says. “However, there are many additional costs that are incurred. For example, you have to crossmatch when you give a person a blood transfusion. You have to do tests to make sure that the blood will be compatible… These can add an additional $200 to $400, so the actual cost [of a blood transfusion] can go up to $400 or $600.”
Infrastructure is also a major hindrance to efficient blood transfusions within today’s donation system. “Today, blood is a national treasure,” Gargir says. “Countries don’t move blood from one place to the other- other than in extreme circumstances.”
With blood transfusion systems requiring advanced infrastructure developing countries are at a great disadvantage.
RedC Biotech plans to solve the infrastructure problem by producing and testing the blood in regional factories. They want to reduce the need for expensive testing equipment at each donation center. In addition, having a “franchise structure”, with production centers around the world will allow each country to have closer access to blood donations. Gargir says that future plans such as solar-powered refrigeration and the creation of freeze-dried blood may help close the infrastructure gap further.
He estimates that it will be about five years until the product is out on the market, and the company’s target cost “is to produce a blood transfusion at $50.” While there is no doubt that RedC’s is a profitable business that has the potential to revolutionize blood transfusions, the long and arduous scientific process is worth it for another reason:
“Without being too poetic about it, it really means saving millions of people’s lives,” Gargir says. “And then we’ll have a real significance on medicine around the world.”
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer



Facebook comments