People who receive aggressive treatment for certain types of cancers may have fertility problems, and children and teenagers “are often of special concern,” according to the American Cancer Society. Treatments like chemotherapy and radiation therapy can damage sperm-forming cells and result in impaired spermatogenesis, the origin and development of sperm cells within the male reproductive organs.
Israeli scientists led by a research group at the Ben-Gurion University of the Negev (BGU) in Beersheba have developed an innovative platform to create sperm in a laboratory through a microfluidic system, which contains hundreds of microchannels for fluids to pass through. The sperm was grown on a special silicon chip developed in collaboration with researchers at the Technion – Israel Institute of Technology. A 3D system was built and integrated to allow the addition of testicular tissue cells. The chip enabled the researchers to grow cells from the testis in the microchip and add fresh cell culture media designed to support cellular growth.
Their research was published recently in the peer-reviewed journal Biofabrication under the title, “Testis on a chip — a microfluidic three-dimensional culture system for the development of spermatogenesis in-vitro.”
Prof. Mahmoud Huleihel from the Shraga Segal Department of Microbiology, Immunology, and Genetics, in the Faculty of Health Sciences at Ben-Gurion University tells NoCamels there was a need to find a method to produce sperm cells in the laboratory, especially for younger males who have not developed sperm yet.
According to Huleihel, when his team began studying some 15 years ago, they knew that when cancer affects sperm generation in adults “medical professionals can cryopreserve sperm from those patients and use them in the future before chemotherapy treatment,” he explains, “The problem is with prepubescent boys who do not generate sperm at their age and the question was how can we cryopreserve the sperm, or how we can make preservation of their fertility if it’s possible.”
Like adult males, these younger males have stem cells, or what he refers to as basic germ cells, which can proliferate, propagate, and differentiate later to produce sperm. “These basic cells are present in boys. So the question was, can we use these cells with any kind of technology to produce sperm in the future?”
Young mice that didn’t produce sperm cells yet were a model the researchers used to imitate conditions for the growth of sperm cells in the testicle. Under laboratory conditions, it was possible to develop a procedure to culture testicular cells in an environment similar to a natural environment.
SEE ALSO: Fairtility Offers Better Way To Choose The Right Embryo For IVF
Prof. Huleihel admits that this is something that has been done before by other scientists around the world. The mice were given specific stems cells which in turn produced fertile sperm. In the case of the boys, the group wanted to find a method to produce sperm that bypasses what Huleihel refers to as “limitations” such as the potential return of cancer cells to the patient’s body.
“The problem with the boys is that we were afraid that some of the tissues that we were going to use in the future still have residual cancer cells. So if we inject these cells to the boys, we might restore the cancer,” he says. Today, there aren’t any methods to isolate the stem cells from the cancer cells. So it’s an admittedly big limitation as to why we cannot use some of the technology already successful in the animal model.”
Sign up for our free weekly newsletter
SubscribeRather than trying to isolate stem cells from cancer cells, the BGU researchers decided they would grow their own stem cells using their novel testis-on-a-chip (ToC) platform with a complete 3D system that allowed the addition of growth factors, and cells from the testicles or any other cells from the body.
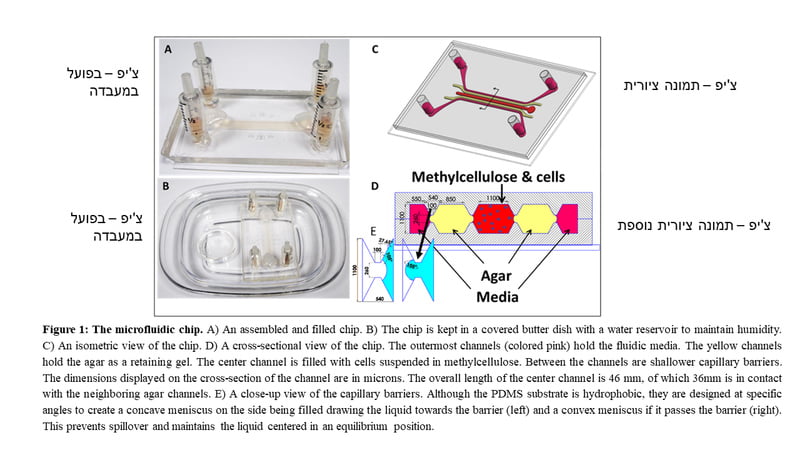
“We thought about some things that we could add in the culture dish to grow with the stem cells,” Huleihel says, “So that we can grow them and use them in differentiation to stem. Then we could isolate these sperm and inject them into a female with limitation of possible cancer restoration.”
The innovative system was successfully tested on sexually young mice that have germ cells for spermatogenesis. The development in the culture was examined after five to seven weeks. Sperm-like structures were found that contain cells in advanced stages (called round spermatids) in the process of sperm formation. The sperm cells reached 95 percent viability, according to the study. The researchers found that the round spermatids eventually fertilized normally and produce offspring.
The research group is now preparing for the next phase of applying the experiment to cells from humans, Prof. Huleihel tells NoCamels. He gives NoCamels a rough estimate of this testing on humans starting in the next two or three years.
“It is very important that this system be optimized using the animal model. And the next step is to use humans to see if we reach the same stage. If the success in the human is similar to what happened in the mouse system — and if they will also become fertile. This is the future stage,” he explains.

He adds, “If we succeed in creating round spermatids, or even complete stem [cells], then we have the patient’s tissue in the future, right? We split this tissue into two things — one that we use for research and the other we keep for the future for these boys.”
The ultimate goal is to use the platform to produce round spermatids that “can fertilize all side, then we can provide it to any hospital,” Huleihel says. “This the aim of science. It’s something that will benefit all of humanity.”
The research group included Prof. Emeritus Eitan Lunenfeld, from the Faculty of Health Sciences at Ben-Gurion University of the Negev and Soroka Medical Center, currently a senior faculty member at Ariel University and Prof. Gilad Yossifon, from the Faculty of Mechanical Engineering at the Technion (currently a faculty member from the School of Mechanical Engineering at Tel Aviv University). The research was led by PhD students Ali AbuMadighem, from the Shraga Segal Department of Microbiology, Immunology and Genetics, Ben-Gurion University of the Negev and Sholom Shuchat from the Department of Mechanical Engineering, Technion – Israel Institute of Technology.
This study was supported by the Israel Science Foundation (ISF) and in collaboration with the Chinese Foundation for Natural Sciences (NSFC) (ISF-NSFC), the Reproduction Hub at the Faculty of Health Sciences, Ben-Gurion University of the Negev, and Council for Higher Education Scholarships for outstanding PhD students from the Ultra-Orthodox and Arab populations.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer



Facebook comments