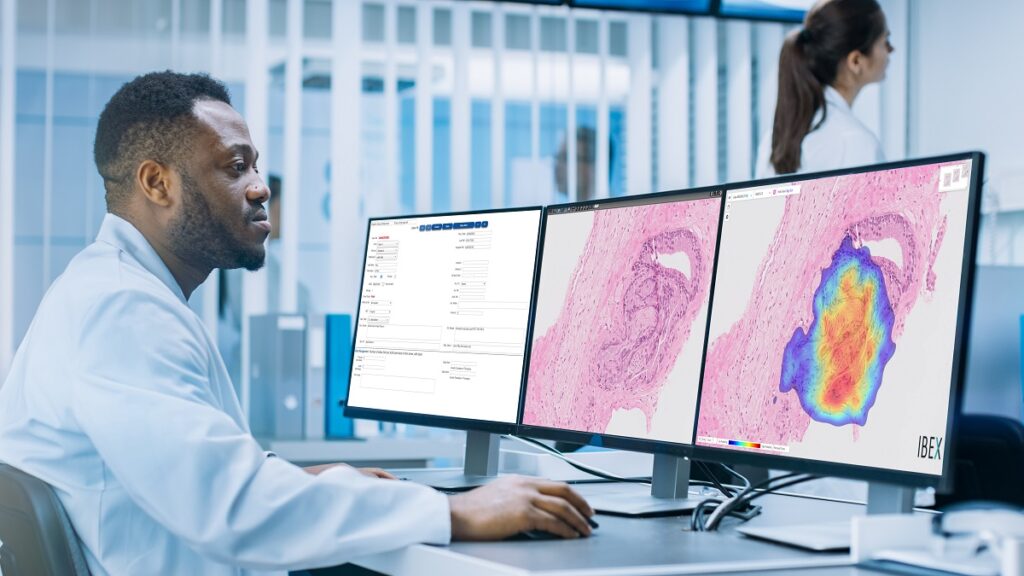
Israeli AI-powered cancer diagnostics company Ibex Medical Analytics announced on Wednesday that it was granted Breakthrough Device Designation by the US Food and Drug Administration (FDA) for its Galen platform that helps pathologists detect and grade cancer in biopsies.
Ibex’s Galen Prostate and Galen Breast are the first AI-powered cancer diagnostics solutions in routine clinical use in pathology and are deployed in labs worldwide. The Galen platform offers the Ibex First Read and the Ibex Second Read, applications that analyze cases prior to human pathologist review, enabling case prioritization, and in parallel with human pathologist review to identify any discrepancies, respectively. The Second Read system for breast and prostate biopsies have been used at the Pathology Institute of Maccabi Health Services – Israel’s leading HMO – since 2018 and the First Read implementation was announced late last year.
The FDA’s Breakthrough Device Designation is granted to technologies that have the potential to provide more effective treatment or diagnosis of life-threatening diseases, such as cancer. Ibex says the designation will enable close collaboration with, and expedited review by the FDA, and provides formal acknowledgment of the Galen platform’s potential benefit as well as the robustness of Ibex’s clinical program.
“Oncology treatments have made great strides, but in order to save more lives it is also essential to see technological advances in cancer diagnostics,” said David Shulkin, MD, former Secretary at the US Department of Veterans Affairs and an advisor to Ibex Medical Analytics. “Enhancing the accuracy of cancer diagnosis and improving the efficiency for the pathologist is paramount to improving quality and affordability of cancer care. Ibex’s AI platform has demonstrated success in helping pathologists worldwide improve care for patients with cancer. This FDA designation is an important step forward in making this technology broadly available in the United States.”
“We are honored to have been granted the Breakthrough Device Designation,” said Ibex CEO and co-founder Joseph Mossel. “Ibex is committed to providing world class tools for pathologists to ensure every patient receives a timely and correct diagnosis, while supporting pathology labs and health systems to increase efficiency and accuracy.
“We are proud to work closely with the FDA and look forward to continuing to collaborate with the agency as we accelerate our clinical program in the United States,” he added.
Ibex Medical Analytics was founded in 2016 as part of the Kamet Ventures incubator and is backed by investors such as Dell Technologies Capital, the corporate venture arm of Dell Technologies, Israeli medtech fund aMoon, Octopus Ventures, Planven Entrepreneur Ventures, and 83North.
SEE ALSO: Israeli AI Diagnostics Firm Ibex Detects Cancer Using Advanced Algorithms
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

Editors’ & Readers’ Choice: 10 Favorite NoCamels Articles

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors



Facebook comments