Israel’s COVID-19 vaccine developer, the government-run bio-defense firm Israel Institute for Biological Research (IIBR), is reportedly aiming to develop a version of the jab that is effective in a single dose while eyeing a redo of the ongoing clinical trials it has already embarked on.
Haaretz reported last week that researchers at IIBR began looking at the possibility of forgoing the second shot if patients are given a higher dose in the initial injection after it became apparent from the first and second phases of the clinical trials on the two-shot BriLife vaccine that this could be feasible.
SEE ALSO: Israeli COVID-19 Vaccine Cleared For 2nd Phase Of Clinical Trial With 1,000 Volunteers
The Ness Ziona-based IIBR launched the second phase of the trial in December with 1,000 volunteers aged 18 and over after the first phase, which began November 1 with 80 volunteers, was completed successfully with no significant side effects. A third phase with up to 30,000 volunteers in Israel and/or abroad was expected to begin this spring once the second stage would prove successful.
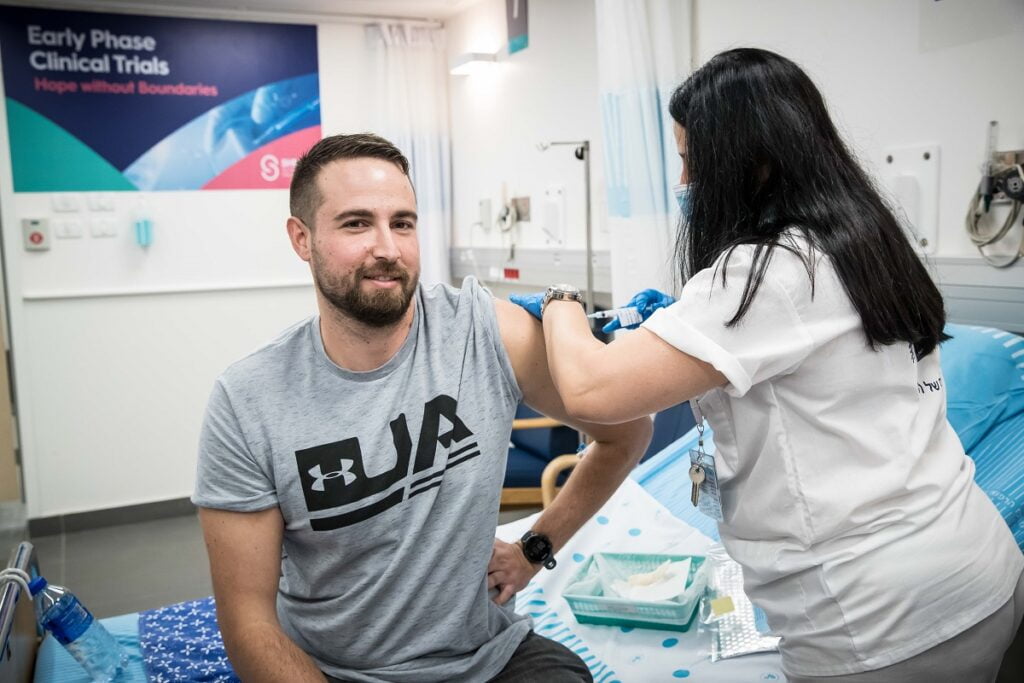
Haaretz further reported that IIBR may move its clinical trials for BriLife from Israel to Argentina whether a third phase is approved for the two-dose injections or a new trial is launched with a single dose. This is due to Israel’s world-leading vaccination rate, with now about 60 percent of Israelis 16 and over having been inoculated.
A delegation headed by physicians from Jerusalem’s Hadassah University Hospital, which is involved in the clinical trial, will likely head to Argentina in the next two weeks to lay the groundwork, according to the report.
The results for Israel’s BriLife vaccine have not yet been made public, so its efficacy against COVID-19 is lesser widely known than established vaccines like Pfizer’s and Moderna’s, but the Israeli trials have involved various doses, Haaretz said, citing unnamed sources.
The Defense Ministry tells NoCamels in a statement that the “clinical trial of the vaccine developed at the biological institute is progressing as planned, and that “there was never a need to repeat the first phase.”
“All volunteers for the second phase of the trial were recruited. The second phase involves examining the number of doses given in two injections,” the statement reads.
“Argentina is being considered as one of the countries where Phase 3 would be carried out,” the ministry adds.
However, Haaretz reported that a Defense Ministry statement to the publication indicated that”an additional, higher dose is, in fact, being considered.”

Israel’s BriLife vaccine used vesicular stomatitis virus (VSV), an animal virus that does not cause disease in humans, and in which the spike protein was replaced with that of SARS-CoV-2, the virus that causes COVID-19. VSV is also the basis for a separate, effective vaccine against the Ebola virus.
Sign up for our free weekly newsletter
Subscribe“In this way, the body thinks it has been infected with the real [corona] virus, but actually it’s just a ‘costume,” said Dr. Hadas, a vaccine developer at the IIBR whose full name was not disclosed, in a video put out by the institute late last year. “So the body develops antibodies against the genetically engineered virus, but in the moment of truth, the body can identify, bind, and neutralize the virus.”
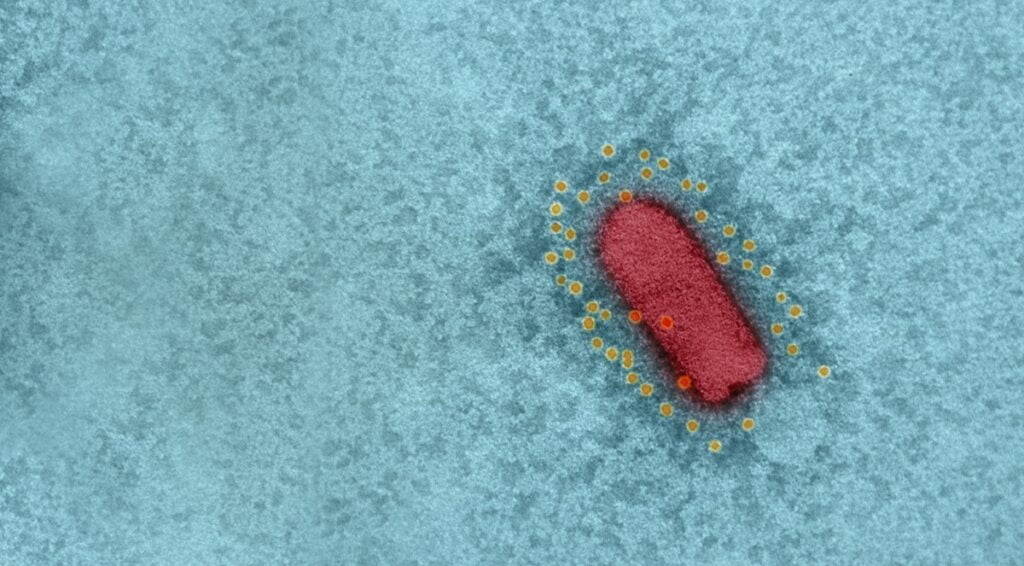
The vaccine, which the scientists called a recombinant VSV-ΔG-spike or rVSV-ΔG-spike, had been tested on a number of animal models, including golden Syrian hamsters, mice, rabbits, and pigs, and was shown to be safe and well-tolerated, and able to bind and neutralize SARS-CoV-2 efficiently.
It differs from an mRNA vaccine, like Pfizer’s and Moderna’s, a type of jab that teaches our cells how to make a protein that triggers an immune response, rather than introducing a weakened or inactivated virus which has been the traditional vaccine approach.
The IIBR said in November that it produced more than 25,000 vaccine doses for the first and second phases and can undertake large-scale production of vaccines – approximately 15 million doses.
Preparation for an unknown threat
The IIBR is a governmental research center specializing in biology, chemistry and environmental sciences and falls under the jurisdiction of the Prime Minister’s Office.
The institute was first established in 1952 and has done extensive research over the years on disease processes, vaccine planning and development, and detection and identification methods. It employs hundreds of scientists, some of them leaders in their fields in Israel and around the world.
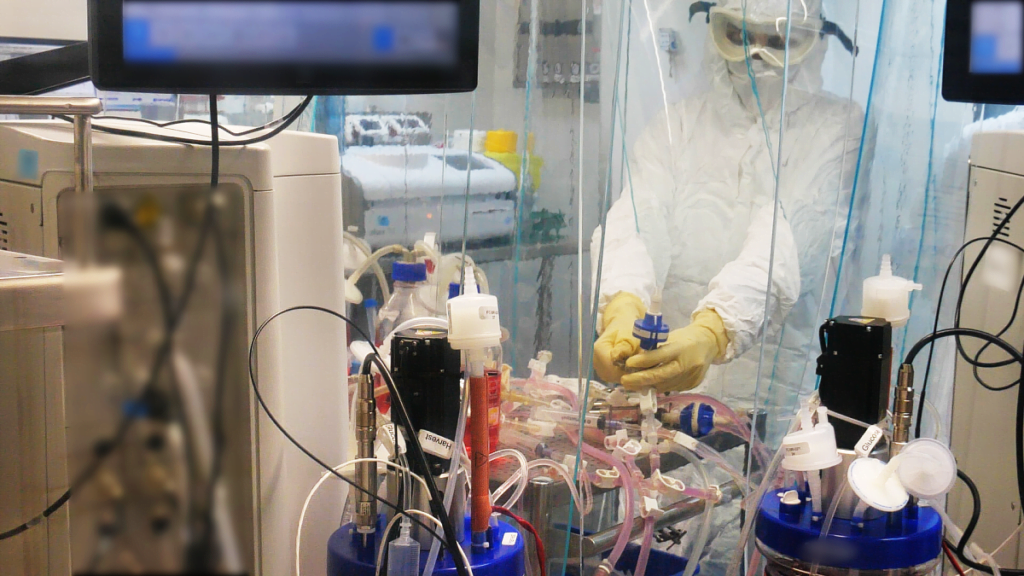
The center said in October that it has been preparing for several years for a scenario with an unknown threat (which it dubbed Unknown X).
“As part of its scientific assessments, the institute purchased and developed advanced platforms, including infrastructure for the rapid identification of epidemic pathogens and tools for the rapid design of effective vaccines in response to outbreaks,” the Defense Ministry said at the time.
Animal models were also put in place to quickly test the safety and efficacy of vaccines and treatments, and new infrastructure were developed for the rapid production of vaccine doses, under stringent regulatory conditions.
NOTE: This article was updated on July 13, 2021 to correct the mistaken assertion that BriLife is an mRNA vaccine. It is a live-virus vaccine based on an existing virus (VSV) that is not harmful to humans.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer




Facebook comments