The announcement last month (first reported by NoCamels) that an Israeli man regained his vision after a decade of blindness following the first surgical implantation of an artificial cornea, made news all over the world.
The surgery, performed at the Rabin Medical Center (also known as Beilinson Hospital), marked a major accomplishment for Israeli medical tech startup CorNeat Vision, the developer of the synthetic cornea used in the procedure, the CorNeat KPro. The company had been working for years to develop the device, and had received the go-ahead for human implantation last summer.
SEE ALSO: Blind Man Regains Sight After Receiving 1st Artificial Cornea Implant By Israel’s CorNeat
The first patient, 78-year-old- Haifa resident Jamal Furani, was also the world’s first person to be successfully implanted with the device as part of an initial trial with 10 pre-approved participants at the Rabin Medical Center. Professor Irit Bahar, director of the Ophthalmology Department, performed the surgical implantation and is expected to conduct subsequent ones.
Following the surgery, a brief period of hospitalization and a few weeks of monitoring for inflammation, Furani was able to read texts and see family members – after years of suffering from vision loss due to edema and other background diseases that damaged his corneas.
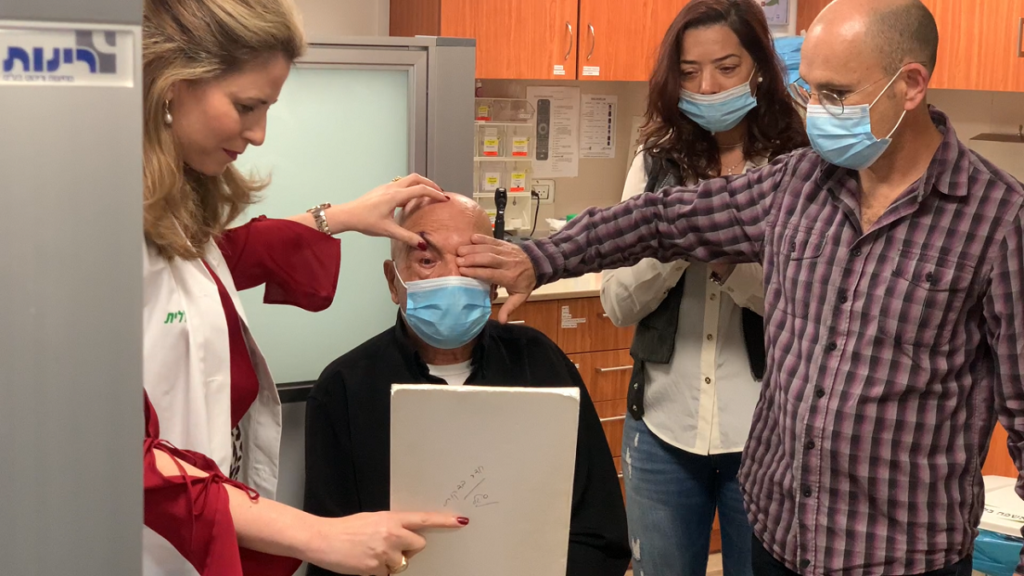
The moment “was very exciting for us; we’ve been trying to create this dream for the past five years and it’s been a rollercoaster,” says CorNeat Co-Founder and Chief Medical Officer Dr. Gilad Litvin, the inventor of the CorNeat KPro. He’d previously said there were “a lot of tears in the room,” after the bandages came off.
Furani’s recovery process involved several weeks of allowing the synthetic material to integrate with his own tissues, a process that had taken up to three months in animal trials, Dr. Litvin explains to NoCamels, and monthly follow-ups.
“There’s a learning curve, we will learn more things about the behavior of the device with more procedures,” he says.
The company is now gearing up for a second implantation procedure in Israel, and the launch of a trial in Canada with 10 patients as well as in six sites at different stages in the approval process in France, the US, and the Netherlands.
At the moment, the clinical trials are for corneally blind patients who are not suitable candidates for – or have failed – one or more corneal transplantation (keratoplasty), or whose conditions make them unsuitable for a transplant due to genetic dispositions, for example.
“The implant is a treatment for people who are blind due to corneal opacity,” Dr. Litvin tells NoCamels. Causes of corneal opacity may include injury, swelling, and infection such as herpes which can have serious complications. Overuse of contact lenses and improper handling may also lead to severe infection and corneal damage.

The CorNeat KPro implant is designed to replace these deformed, scarred, or opacified corneas and fully rehabilitate the vision of corneally blind patients. The implant’s lens integrates with resident ocular tissue using a patented synthetic non-degradable nano-fabric placed under the conjunctiva (the tissue that lines the inside of the eyelid), which stimulates cellular growth and facilitates complete integration with the surrounding tissue.
Current biological solutions are either sutured to or become integrated with native corneal tissue, which lacks blood vessels and heals poorly, Dr. Litvin previously explained to NoCamels.
Treating corneal blindness
Since the announcement of the surgery last month – and Furani’s restored sight – CorNeat has been fielding calls and requests “from people all over the world” – from investors to physicians to people interested in participating in the trials, says Almog Aley-Raz, CorNeat Vision’s co-founder, CEO and VP R&D.
Potential patients are lining up as there is more understanding of the procedure, he tells NoCamels.
Meanwhile, coordinating a multi-national trial effort has required a huge investment of time and resources, and the separate approval processes have been rigorous, Aley-Raz indicates.
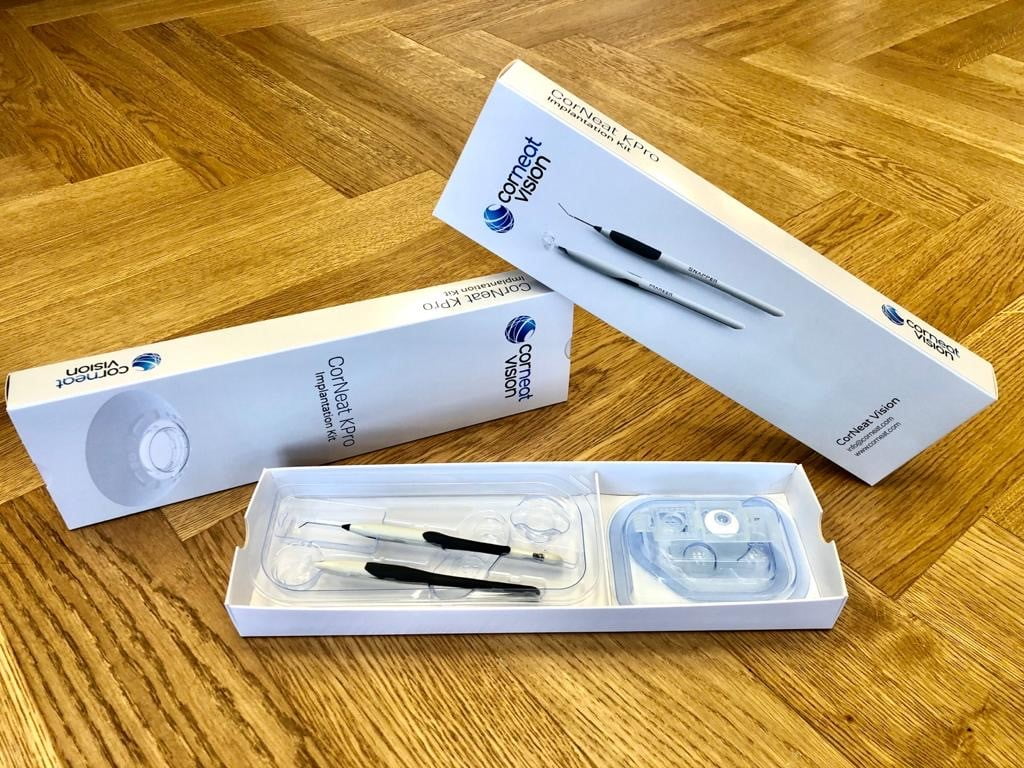
CorNeat, he says, is proud to be able to do everything in-house, dispatching its own clinical department to launch applcations, creating its own database and working methodically to get the required approval.
“This is unique because it gives us control over the whole process and we can address questions and issues with regulators immediately,” Aley-Raz tells NoCamels.
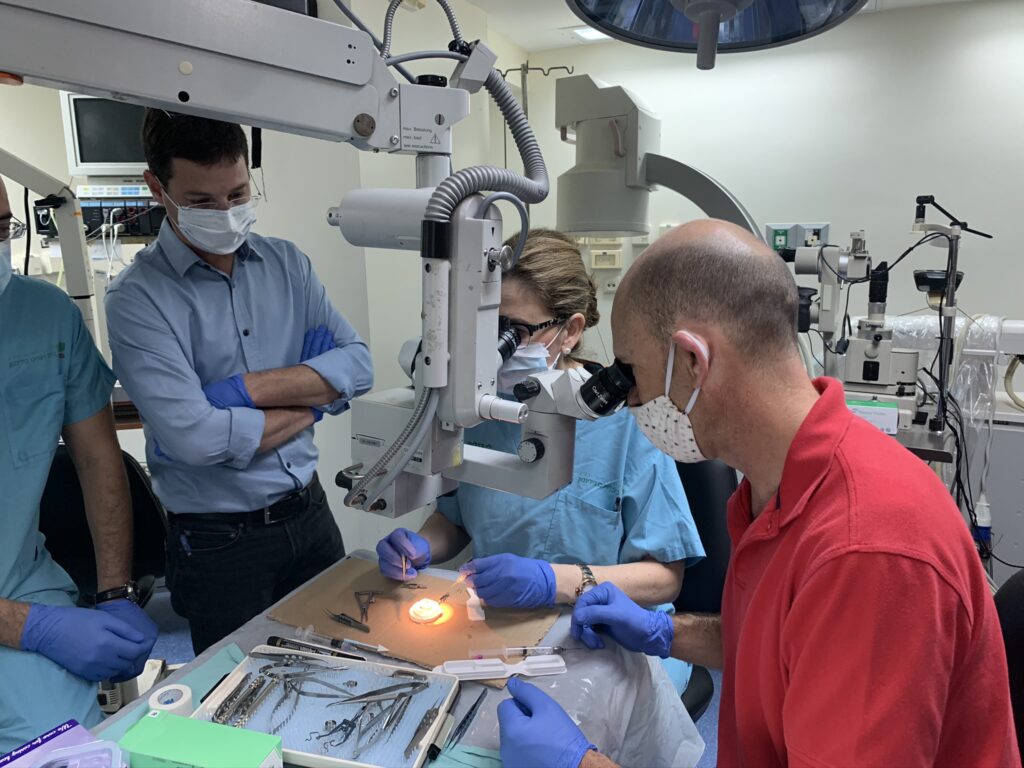
The endeavor is part of CorNeat’s grand vision to solve corneal blindness all over the world and tackle the inherent inequality of access to such healthcare solutions.
“Over 50 percent of people in the world have no access to corneal tissue, and corneal banks, and no access to synthetic solutions,” Dr. Litvin says. Every year, the number grows because of a bottleneck created by the gap between the number of people who become blind and the number of people who can be treated.
According to the World Health Organization, approximately two million new cases of corneal blindness are reported each year, and 30 million people worldwide are legally blind in one or both eyes from corneal injury and disease. A recent study published JAMA Ophthalmology found that the shortage of corneas is around one available cornea for every 70 patients.
Sign up for our free weekly newsletter
SubscribeIn China, with over five million patients waiting for keratoplasty and only a few thousand transplants a year available, CorNeat is planning a study this year with 60-70 local patients to facilitate turning the CorNeat KPro into a primary solution for corneal blindness, Dr. Litvin says.
“Our mission is to promote equality by restoring vision,” he adds. “We wanted to create a device that doesn’t need any tissue, so you could have a completely synthetic alternative to human donor tissue.
In developed countries, Aley-Raz and Dr. Litvin believe the cost of the implant will be covered by insurance companies and may become part of the “healthcare basket” in states with universal healthcare.
Elsewhere, they estimate the cost at $2,000-$3,000 but say that they are working to reduce the cost of production and will leverage different country programs that aim to reduce blindness.
“The cost of blindness is already so high; it’s not just about the social impact,” says Aley-Raz.
CorNeat’s solution ‘suite’
CorNeat not only developed the innovative implant, it has also built an on-demand virtual training solution for surgeons that has come in handy during COVID-19.
The company wanted to create a procedure that was “very approachable” and simple to teach in about two days of training, Dr. Litvin tells NoCamels.
The solution involves special headsets and an HD live stream with latency of about one second that CorNeat calls RSVP – remote surgeon virtual presence, says Aley-Raz.
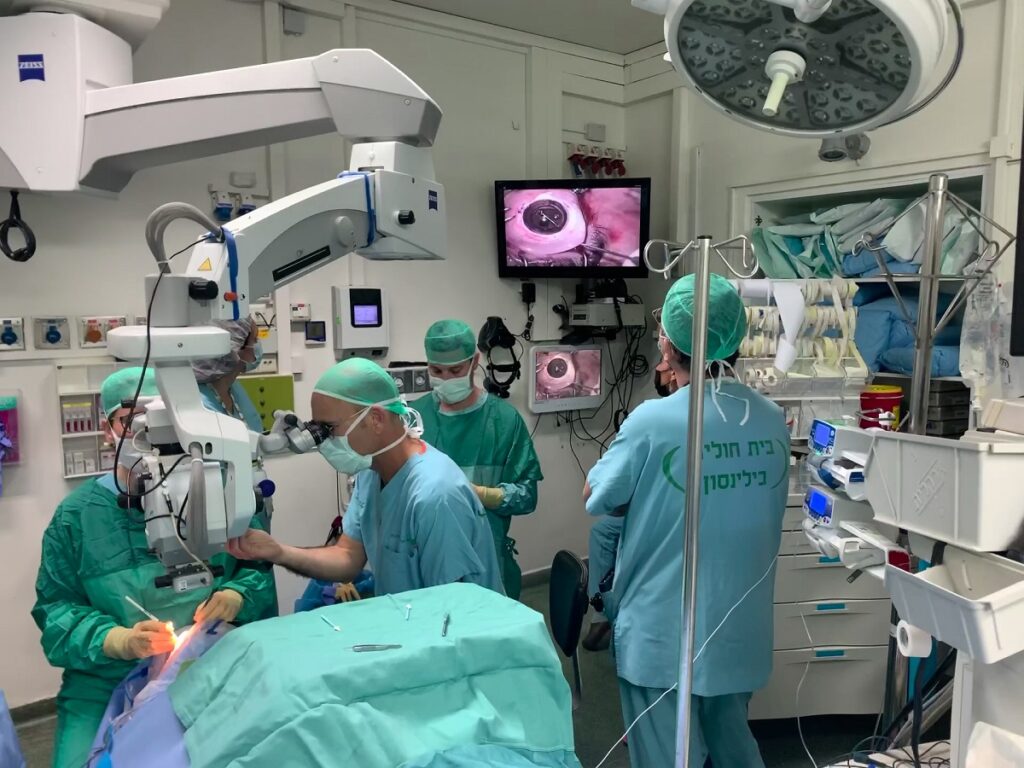
“There are lots of details and explanations and live instruction,” Dr. Litvin adds.
SEE ALSO: Israeli Medtech Startup CorNeat Vision Uses Patented Implants To Treat Blinding Diseases
By contrast, he noted how, in order to properly transplant corneas, ophthalmologists must specialize in corneal transplantation, a procedure that takes at least a year or two to learn. Additionally, once a cornea is harvested, ophthalmologists have only one week to implant it, whereas CorNeat’s technology has a shelf life of two years.
A mountain biking trip
Aley-Raz and Dr. Litvin met by chance during a mountain biking trip in Spain several years ago with their respective kids, who are close in age.
The two struck up a conversation about their careers – Dr. Litvin was working as an ophthalmologist and a surgeon, while Alez-Raz was in the high-tech industry – and the rest, as they say, is history.
They founded CorNeat Vision in 2015 in the central Israeli city of Ra’anana and met with investors but ended up funding their pilot themselves.

Over the years, they’ve relied on their respective strengths and gained new skills and insights.
“Gilad [Litvin] became a businessman,” laughs Aley-Raz, “but he still performs surgery once a week and having an active surgeon in a startup is very unique.”
“I definitely wanted to walk this path with Almog [Alez-Raz],” Dr. Litvin tells NoCamels.
“And it’s all because of a vacation in Spain,” he says.
Today, CorNeat offers a number of solutions in addition to the CorNeat KPro, all aimed at treating blinding diseases.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer




Facebook comments