Early scientific research has shown that an existing cholesterol drug may help lessen the dangers of the COVID-19 disease, downgrading it to the threat of a common cold. This is according to a new study by Israeli and American scientists.
In lab tests, the cholesterol-lowering, FDA-approved drug Fenofibrate (Tricor) showed “extremely promising results” by interfering with how the SARS-CoV-2 virus – the novel coronavirus that causes COVID-19 – is able to reproduce, according to the research.
The study was led by the Hebrew University of Jerusalem’s Professor Yaakov Nahmias, an Israeli biomedical engineer and entrepreneur, and Dr. Benjamin tenOever, a professor of medicine and microbiology. Dr. tenOever is also the director of the Virus Engineering Center for Therapeutics and Research at Mount Sinai Medical Center’s Icahn School of Medicine in New York.
The study titled “The SARS-CoV-2 Transcriptional Metabolic Signature in Lung Epithelium” appeared in the Sneak Peek section of the scientific journal Cell Press earlier this month. It is currently under review.
Researching how the virus reproduces
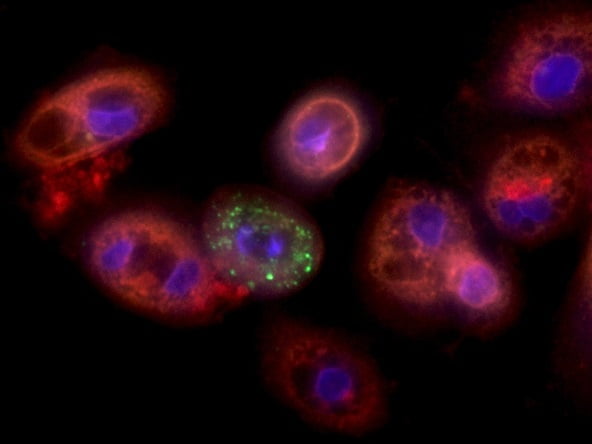
Over three months, Professor Nahmias and Dr. tenOever conducted research on how the SARS-CoV-2 virus changes patients’ lungs to reproduce itself. They found that the virus prevents the routine burning of carbohydrates, resulting in large amounts of fat accumulating inside lung cells. This is a condition the virus needs to reproduce, the Hebrew University explained in a statement.

With this information, they began to screen FDA-approved medications that inhibit the virus’ reproductive process and found initial success with Fenofibrate (Tricor) in vitro. By allowing the cells to burn more fat, the drug breaks the virus’ grip on the cells and prevents SARS CoV-2’s ability to reproduce.
According to the study, within just five days of treatment, the virus “almost completely disappeared,” according to the Hebrew University.
This understanding of how SARS CoV-2 behaves may help explain why patients with medical conditions such as elevated blood glucose and heart disease often run a higher risk of developing severe cases of COVID-19.
“By understanding how the SARS-CoV-2 controls our metabolism, we can wrestle back control from the virus and deprive it of the very resources it needs to survive,” Professor Nahmias said in the university statement.
“With second-wave infections spiking in countries across the globe, these findings couldn’t come at a better time,” Nahmias added, hailing global cooperation for a treatment or cure.
Professor tenOever said the collaboration “demonstrates the power of adopting a multi-disciplinary approach to study SARS-CoV-2 and that our findings could truly make a significant difference in reducing the global burden of COVID-19.”
Sign up for our free weekly newsletter
SubscribeAs the research progresses, “this course of treatment could potentially downgrade COVID-19’s severity into nothing worse than a common cold,” Professor Nahmias offered.
Professor tenOever is advocating caution. He said that “while Fenofibrate does work very well at inhibiting SARSCoV-2 in cells in a Petri dish, this is true for many compounds. It needs to work in vivo before anyone should get excited.”
The race for a coronavirus treatment or vaccine
Close to 200 teams worldwide are working to develop a vaccine or a treatment for COVID-19. Twenty-five are currently under clinical evaluation. These include a promising vaccine candidate developed by Massachusetts-based company Moderna, which is currently in Phase III trials..
Another promising vaccine candidate, also in Phase III trials, was developed by the University of Oxford which recently signed a distribution agreement with drugmaker AstraZeneca.
Last month, Israeli researchers from the government-run Israel Institute for Biological Research (IIBR) indicated that a vaccine they developed for SARS CoV-2 was found to be effective in trials involving hamsters, paving the way for testing with humans.
The IIBR, a research center specializing in biology, chemistry and environmental sciences that falls under the jurisdiction of the Prime Minister’s Office, was first tapped by PM Benjamin Netanyahu in early February to begin development on a vaccine.

In early April, the center reported “significant progress” on the vaccine and initial trials on rodents. The secretive institute, based in Ness Ziona, has also been working on researching potential treatments and in early May announced that it made a breakthrough on an antibody that neutralizes the virus. That same month, it further announced that a combination of two existing antiviral drugs for Gaucher disease appears to inhibit the growth of SARS CoV-2, and may work against other viral infections, including a common flu strain.
According to the researchers’ most recent findings on a vaccine, a single dose was able to “protect hamsters against SARS-CoV-2” and showed “rapid and potent induction of neutralizing antibodies against SARS-CoV-2.”
In late April, Israeli scientists at the Migal Galilee Research Institute formed a new company, MigVax, to further adapt a vaccine they developed for a deadly coronavirus affecting poultry for human use. The scientists had been working for four years to develop a vaccine for IBV (Infectious Bronchitis Virus) which affects the respiratory tract, gut, kidney and reproductive systems of domestic fowl.
MigVax raised $12 million in an investment round led by OurCrowd for further development of the vaccine. The startup hopes to begin clinical trials this summer.
Related posts

Editors’ & Readers’ Choice: 10 Favorite NoCamels Articles

Forward Facing: What Does The Future Hold For Israeli High-Tech?

Impact Innovation: Israeli Startups That Could Shape Our Future





Facebook comments