Israeli medical technology company BrainsWay was selected by the US Food and Drug Administration (FDA) as one of eight companies worldwide to participate in an innovation challenge tackling the opioid epidemic raging across the country.
The challenge was first announced in May, and the eight companies were chosen this week among 250 to develop medical devices that would help predict the misuse of opioids, detect an overdose, or help treat the addiction.
“The opioid epidemic is one of the most profound public health crises facing the United States and the current crisis of opioid overdose deaths requires innovative approaches,” the FDA wrote at the time.
SEE ALSO: This Israeli-Developed App Can Save You From Anaphylactic Shock – Or An Opioid Overdose
The agency said the medical devices from the selected participants “include those intended to predict the risk of opioid used disorder (OUD), detect opioid overdose, dispense medication and provide pain treatment alternatives to opioids.”
BrainsWay, founded in 2003, develops Deep Transcranial Magnetic Stimulation devices (DTMS) for non-invasive activation of deep brain structures that can cover a broad range of brain disorders. In August, the company received initial FDA approval for its non-invasive device in the treatment of obsessive-compulsive disorder (OCD), and was previously cleared in May for the treatment of Major Depressive Disorder (MDD).
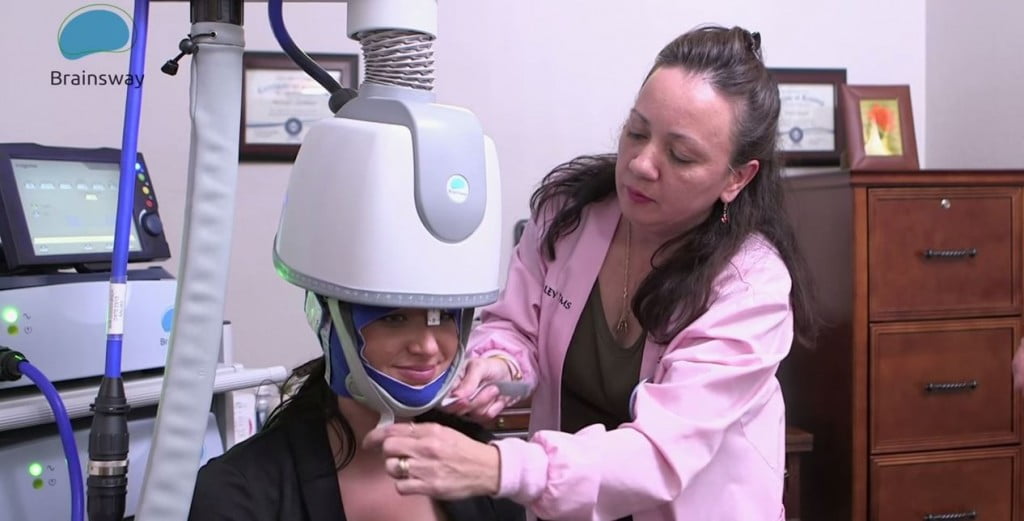
BrainsWay treatment. Courtesy
According to the FDA, BrainsWay is set to develop a device for opioid use disorder therapy. The seven other companies, a majority US-based, will develop systems for pain therapy, medication dispensation, overdose detection, drug screening, and virtual reality (VR) treatments for chronic pain.
Medical devices at any stage of development were eligible for the challenge.
The opioid crisis in the US has garnered international attention for its startling statistics. According to the US National Institute on Drug Abuse, 115 Americans die every day from an opioid overdose.
More than 40 percent of all US opioid overdose deaths in 2016 involved a prescription opioid, according to the Centers for Disease Control and Prevention. Overdose rates from prescription opioids were highest among people aged 25 to 54 years, according to the report. And based on data from Substance Abuse and Mental Health Services Administration’s national survey on drug use and health, 11.1 million people aged 12 and older had misused prescription pain relievers in 2017.
Sign up for our free weekly newsletter
Subscribe
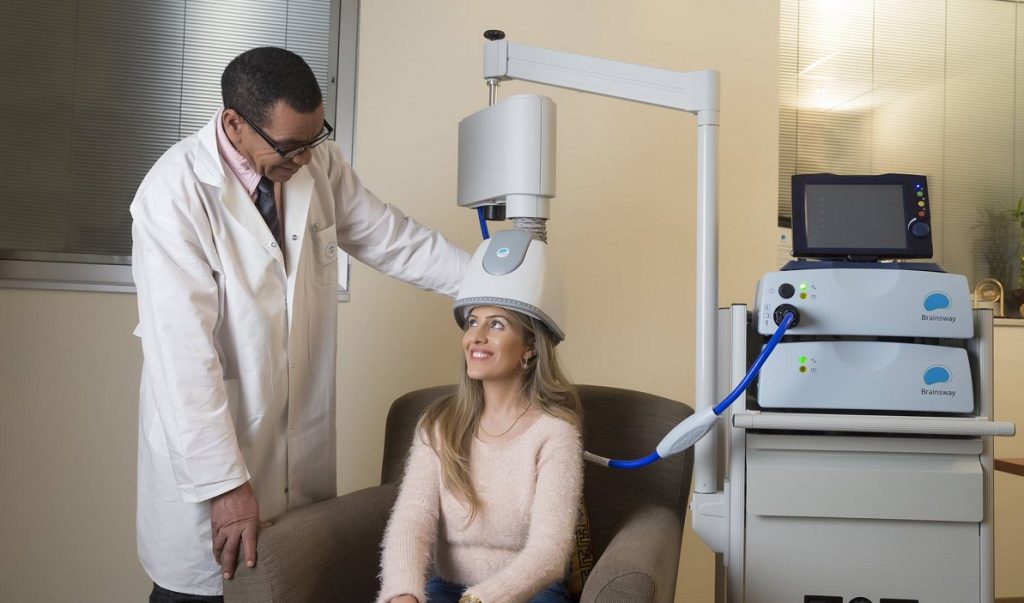
A BrainsWay helmet. Courtesy
The eight companies selected for the FDA innovation challenge will work closely with the agency to “accelerate the development and expedite marketing application review of innovative products, similar to what occurs under the Breakthrough Devices Program“, which helps expedite “certain medical devices that demonstrate the potential to address unmet medical needs for life-threatening or irreversibly debilitating diseases for which no approved or cleared treatment exists or that offer significant advantages over existing approved or cleared alternatives.”
The companies will enter a 90-day collaboration to develop mutual understanding of the product profile including the patient and user needs, and the important risks and benefits, and to discuss the potential regulatory pathways going forward.
“While these products will not automatically receive marketing authorization from the FDA, the device developers will receive increased interaction with CDRH experts, guidance for clinical trial development plans, and expedited review,” wrote Drs. Jeffrey Shuren and Jonathan Jarow, director and chief medical officer respectively of the FDA’s Center for Devices and Radiological Health (CDRH), wrote in a post announcing the winners.
“We believe the greatest opportunities for medical devices to help prevent opioid use disorder are devices that could help identify people likely to become addicted, devices that manage pain as an alternative to opioids or reduce the need for opioid medications,” they wrote.
The CDRH has cleared, granted, or approved more than 200 devices related to the treatment or management of pain, including 10 with new or novel technologies…which may reduce the need to administer opioid drugs to patients suffering from either acute or chronic pain, they said.
BrainsWay was founded by Abraham Zangen, Yiftach Roth, Avner Hagai and David Zacut and has been listed on the Tel Aviv stock exchange since 2007.
The company has been developing a host of treatment devices, including for obesity, schizophrenia, and smoking cessation. BrainsWay says it has treated almost 40,000 patients across 17 countries with its devices.
This challenge is BrainsWay’s first foray into treating opioid addiction.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer



Facebook comments