Now here’s a breath of fresh air! A solution to replace asthma inhalers has yet to be invented, but studies show that with regular breathing exercises, dependency on inhalers and other devices can be greatly decreased.
With a name inspired by ‘alveoli’ – the air sacs found in human lungs where inhaled oxygen passes through – Alvio is the first line of products created by Israeli entrepreneur Bezalel Arkush’s company Quality of Life (QoL) that relies on natural methods to help people improve their overall respiratory state.
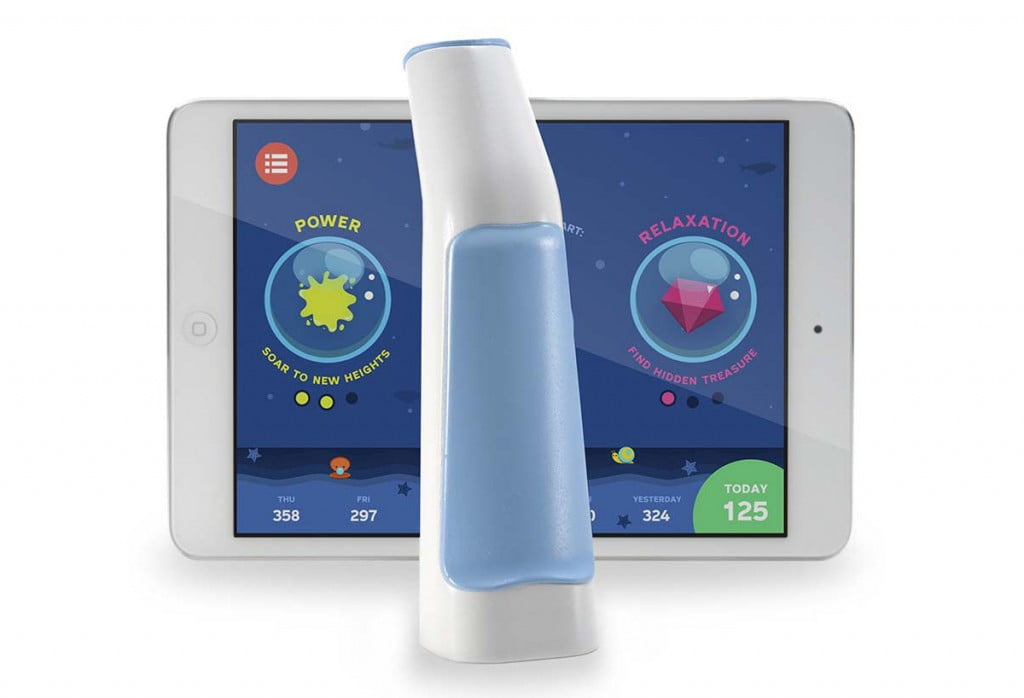
Created by Arkush in 2012, Alvio is the world’s first integrative breathing trainer that teaches asmatics proper breathing techniques with fun, intuitive games. Like a number of other medical devices that have come to embrace the benefits of internet connectivity, Alvio ‘breathes’ new life into current respiratory training by offering interactive elements and automatically collecting data to inform users of their progess. Alvio consists of a standard breathing device and a complimentary app, elements that work together to create a personalized respiratory training schedule that claims to naturally reduce asthmatic breathing patterns.
The healthy habits children won’t know they’re learning
Breathing trainers not only help people with common respiratory problems like asthma or chronic obstructive pulmonary disease (COPD) develop stronger lungs, they are also believed to benefit athletes, musicians, opera singers and even patients under post-operative care after surgery. But as Alvio CEO Bezela Arkush recognized in the case of one of his own friends using an incentive spirometer after surgery, current breathing trainers are not as engaging as they are easy to use.
SEE ALSO: Researchers Identify Possible Root Of Allergies
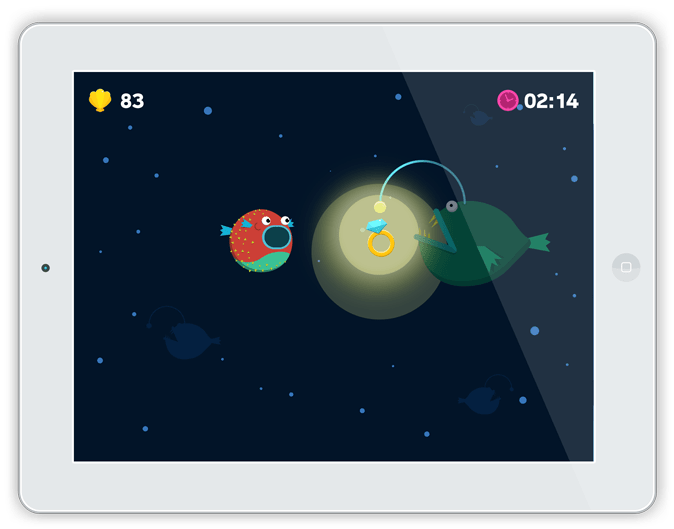
Arkush realized that this is especially problematic for children because they tend to shy away from routine and require a good deal of outside stimulation to want to continue practicing correct breathing methods. In addition, the earlier children get into the habit of practicing their breathing exercises, the less reliant they ultimately become on inhalers. For concerned parents, Alvio disguises routine with a fun, award-incentivizing mobile game that was specifically designed with doctors to facilitate appropriate breathing exercises. In addition, Alvio’s app tracks the child’s breathing progress as they play, making this data available to both parents and healthcare providers to ensure proper care.
“One of the biggest challenges in respiratory issues is how to monitor your status to avoid hospitalization,” Arkush tells NoCamels. “One of the basic things you can do is monitor your state.”
Alvio tracks the breathing health of a user with every use of its breathing device, recording breathing performance over time. By giving parents a way to monitor their child’s respiratory condition closely, they have more peace of mind to let their ‘kids be kids.’
Sign up for our free weekly newsletter
SubscribeAdults too can ‘breathe easy’
Grown adults with respiratory issues may not need app games to help them develop solid breathing habits, but they too can benefit from Alvio’s monitoring feature. According to Arksuh, QoL does intend to develop alternative versions of Alvio that are better suited for adults. Even for patients that do not personally own a smartphone, Alvio provides a breathing device with an additional screen feature.
SEE ALSO: NaNose: The Breathalyzer Test That Sniffs Out Lung Cancer Before It Spreads
As of December 2013, R/GA Ventures, an award-winning digital agency, and Techstar, both invested in QoL. Alvio also receives funding from several undisclosed private investors, as well as from an international American rehabilitation center that hopes to be among Alvio’s biggest customers. In June, the company received a $100,000 grant from Pilot Health Tech NYC and has participated in the well-known TEDMED talks on medical innovation.
With the QoL headquarters in New York City, Alvio’s initial focus will be on the American market. In addition to carrying out several clinical tests at Montefiore Medical Center in NYC, Alvio continues to maintain good relationships with local, supportive hospitals, awaiting US Food and Drug Administration approval for its device.
According to Arkush, Alvio will be made available to the consumer market within the first quarter of 2015, at a starting price near $200 USD. Orders can be made on Alvio’s website, and the company is looking into collaborating with distributors, though it is set to encounter difficulty until it receives FDA approval.
“Asthma is not a curable disease,” Arkush reiterates. “It’s a chronic condition that you constantly have to monitor and improve.” Alvio was designed to help ease the lifestyles of individuals, specifically children, that struggle with dehabilitating respiratory problems, showing them that asthma be beat, one breath at a time.
Photos: Alvio
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer



Facebook comments