The American Food and Drug Administration (FDA) has approved the marketing of a device that detects life-threatening cerebral hemorrhages without the need for CT Scans. The device will therefore prevent unnecessary radiation emitted by the scans.
Cerebral hemorrhages occur when blood vessels are torn inside the brain or between the brain and the skull. With the spread of the bleeding, the soft brain tissues are squeezed which can lead to headaches, vomiting, dizziness, drowsiness, weakness and seizures to the point of unconsciousness. An intracranial hemorrhage is a life threatening condition if not treated immediately. Currently the only way to verify whether there is bleeding inside the brain is by way of CT scan, which emits high amounts of radiation. That is why doctors are often faced with a dilemma, especially when children are involved and when the situation is not unambiguous.
Infrared light
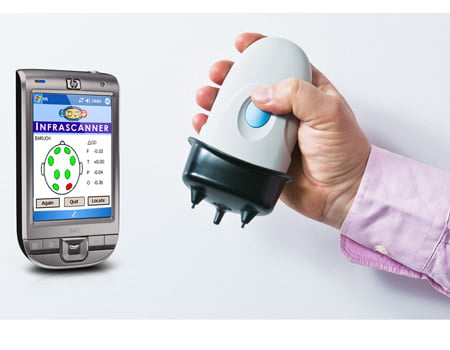
This new device was developed by Dr. Baruch Ben-Dor, CEO of the company InfraScan. The device, called “Infrascanner 1000”, uses an infrared light with a wave-length that can penetrate tissues and bones. The technology works by analyzing light: Blood collected inside the brain following a cerebral hemorrhages absorbs light differently than the rest of the brain tissues.
InfraScan says: “When additional underlying extra vascular blood is present due to internal bleeding, there is a greater local concentration of hemoglobin and consequently the absorbance of the light is greater while the reflected component is less.”
In addition, by comparing the optical density from different areas of the skull, doctors can use the information to know whether there is a high risk of a cerebral bleeding. If such a risk exists, then the doctor can send his or her patient for a more in-depth CT scan.
Non-invasive hand-held device
“Our scanner is a hand-held, non-invasive, near-infrared based mobile imaging device to detect brain hematoma at the site of injury within the ‘golden hour.’ This refers to the period following head trauma when pre-hospital analysis is needed to rapidly assess the neurological condition of a victim,” says InfraScan.
The FDA reviewed the research conducted with the device on which 383 adults.The findings revealed that the device has a 75 percent accuracy rate in detecting bleedings in the brain. Another research, conducted in Moscow with infants, toddlers and children, had an accuracy rate of nearly 90 percent.
InfraScan says: “The scanner will be an affordable, accurate and clinically effective screening solution for head trauma patients in settings where timely triage is critical.”
Photo courtesy of InfraScan
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer




Facebook comments