Scientists have identified a mutation that might underlie an extremely rare condition, called “adermatoglyphia,” which causes people to be born without any fingerprints.
The research, published by Cell Press online August 4th in the American Journal of Human Genetics, not only provides valuable insight into the genetic basis of adermatoglyphia and of typical fingerprint formation but also underscores the usefulness of rare genetic mutations as a tool for investigating unknown aspects of our biology.
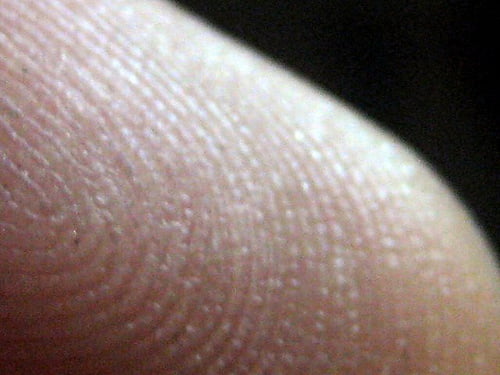
Human skin has ridges called dermatoglyphs that are present on the fingers, palms, toes and soles. The dermatoglyphs on the finger tips, better known as fingerprints, are often used as a means for establishing identity. In fact, adermatoglyphia was recently named “immigration delay disease” because affected individuals report significant difficulties entering countries that require fingerprint recording.
“We know that fingerprints are fully formed by 24 weeks after fertilization and do not undergo any modification throughout life,” explains the senior study author, Dr. Eli Sprecher from Tel Aviv Sourasky Medical Center in Israel. “However, the factors underlying the formation and pattern of fingerprints during embryonic development are largely unknown.”
To better understand the genetics of fingerprint formation, Dr. Sprecher and colleagues investigated a large Swiss family presenting adermatoglyphia. All affected members of the family had displayed an absence of fingerprints since birth, and this absence was associated with a reduced number of sweat glands.
Using a sophisticated genetic analysis of affected and unaffected family members, the researchers discovered that a mutation in the gene SMARCAD1 causes the disease. The protein encoded by the gene is thought to control the expression of a large number of target genes associated with development. More specifically, the group demonstrated the existence of a short version of SMARCAD1 that was exclusively expressed in the skin and was mutated in individuals with the disease.
“Taken together, our findings implicate a skin-specific version of SMARCAD1 in the regulation of fingerprint development,” concludes Dr. Sprecher. “Although little is known about the function of full-length SMARCAD1 and virtually nothing regarding the physiological role of the skin-specific version of the gene, it is tempting to speculate that SMARCAD1 in the skin may target genes involved in dermatoglyph and sweat gland development, two structures jointly affected in the present family.
Further, as abnormal fingerprints are known to sometimes herald severe disorders, our finding may also impact the understanding of additional diseases affecting not only the skin.”
Photo by RandomShoox
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer



Facebook comments