This article was first published by The Times of Israel and is re-posted with permission.
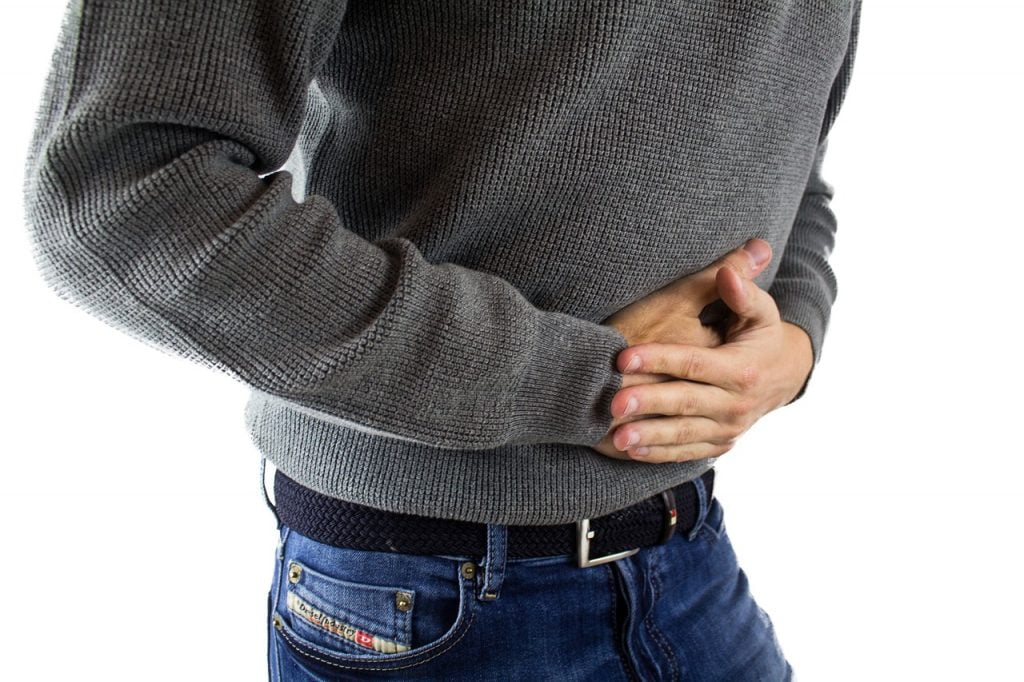
RedHill Biopharma Ltd. said on Monday that a late-stage clinical trial of its drug to treat Crohn’s disease found the medication to have positive safety and efficacy results.
The biopharmaceutical firm said that the results “demonstrated [the] superiority of RHB-104 over a placebo in achieving remission at week 26,” the company said in a statement.
The company has developed RB-104 to treat Crohn’s, a severe inflammatory affliction of the gastrointestinal tract that has no known cure.
The potentially groundbreaking orally administered drug combines three kinds of antibiotics, and is based on the hypothesis that Crohn’s disease is caused by a bacterial infection called the mycobacterium avium subspecies paratuberculosis (MAP) bacteria, which has been found in patients suffering from Crohn’s.
“The robust results of this study demonstrate that RHB-104 could become a leading therapeutic option in Crohn’s disease and bring hope to patients worldwide,” said Ira Kalfus, RedHill’s medical director, in the statement. “The availability of antibiotic therapy for treating Crohn’s disease could be transformative.”
Patients treated with the drug also showed a statistically significant benefit in reaching early remission at week 16, and durable remission in weeks 16-52, the statement said.
To read the full article, click here.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer




Facebook comments