Every person who has undergone surgery knows the discomfort that comes with stitches and the aesthetically displeasing scars that result from them. They may be relieved to hear an Israeli medical company has come up with a way to close incisions using plasma instead of a needle and thread.
Plasma is one of the four fundamental states of matter (the others being solid, liquid and gas). Studies have shown that it has the potential to disinfect, control bleeding, treat burns, and weld tissue in surgical situations.
Despite these benefits, there has been only limited use of plasma because of the high temperatures it must be operated in – which can have harmful effects on the body’s tissues.
Related articles
- InSightec’s Solution Will ‘Cook’ And Destroy Tumors In the Body
- Mazor Robotics: Revolutionizing The World Of Surgery With Robot Side-Kicks
IonMed is trying to change this with their innovative “cold plasma” technology, BioWeld1. This tool utilizes plasma at 40 degrees Celsius and is safe to use on the human body. The welding procedure takes only a few minutes and seals the area completely. According to IonMed, BioWeld1 leaves only minimal scarring and does not require complex training to operate.
No sutures or staples needed
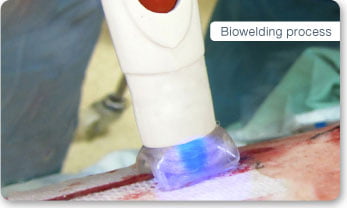 The company has so far had three successful clinical trials to test their product. In the most recent trial, BioWeld1 was used to close the skin incisions of 16 women who underwent Caesarean sections. No sutures or staples were used to close the skin, and yet several weeks following the procedure the wounds had healed and the likelihood of complications such as infection were very low, the company reported.
The company has so far had three successful clinical trials to test their product. In the most recent trial, BioWeld1 was used to close the skin incisions of 16 women who underwent Caesarean sections. No sutures or staples were used to close the skin, and yet several weeks following the procedure the wounds had healed and the likelihood of complications such as infection were very low, the company reported.
“I am very pleased with the study’s findings and our system’s performance,” IonMed’s CEO Amnon Lam said. “We believe that our technology may assist in key applications such as tissue welding, intestinal anastomosis, and treatment of burns and chronic wounds.”
IonMed began developing BioWeld1 in 2009 from their base in Yokneam, Israel. Lam developed the product after extensive testing in different fields of health care. He and his brother, Ronen, felt that wound closure followed outdated procedures that they could radically change with BioWeld1.
The Lams are now in the process of seeking series B funding. The company anticipates receiving the CE mark of approval in Europe for their technology by the end of the year. After closing its next financial round, IonMed will begin a round of trials in Europe and the United States in order to receive approval from the US Food and Drug Administration (FDA) and launch its next cold plasma-based product.
Photo: Group of surgeons by Bigstock
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer




Facebook comments