When Israeli entrepreneur Alon Ironi founded biomedical company Theranica Bioelectronics in 2016, the last migraine medication to have hit the market emerged in the 1990s, and the migraine treatment world was largely static.
Remedies for migraines – throbbing or pulsating headaches that can be severely painful, often debilitating, and may be accompanied by nausea and vomiting – have included prescription drugs like triptans and ergots, as well as home remedies. But not all sufferers respond to these treatments. According to the American Migraine Foundation, there is no one treatment that has been proven to work – and work for everyone.
Theranica proposes a different path to help migraine sufferers and its offering is so promising, it has been named as one of 36 game-changing companies in 2020 by New York-based research firm CB Insights – the only Israeli company on the list – and among TIME magazine’s best inventions of 2019 (alongside eight other Israeli innovations).
Neuromodulation therapy
The Netanya-based company specializes in combining neuromodulation therapy with wireless technology for pain mitigation and develops wearable devices that address prevalent conditions, such as migraines. In essence it offers a drug-free future for chronic illness.

Headache disorders are among the most common disorders of the nervous system. Migraines themselves affect one in seven people worldwide, according a 2013 study published in the Journal of Headache and Pain, a peer-reviewed medical journal that covers research on headache and related types of pain. They are more common in women than men and are caused by the activation “of a mechanism deep in the brain that leads to [the] release of pain-producing inflammatory substances around the nerves and blood vessels of the head,” according to the World Health Organization. Many of those affected – as anyone who suffers from migraines will attest – can experience severe impairment during an attack as they can cause visual, sensory, motor or verbal disturbance (also called migraine aura).
Theranica’s wearable device, the Nerivio, is poised to disrupt this industry and can potentially offer much-needed relief for millions of people. It is indicated for acute treatment of migraine (with or without aura) in adults who do not suffer from chronic migraines.
Ironi, Therania’s co-founder, CEO, and president, tells NoCamels that the idea behind the Nerivio began with a different kind of pain.
“With a history of back pain, I was introduced to devices for pain relief based on neuromodulation that were expensive and complicated. I dived into neurology and pain theory and was introduced to Professor David Yarnitsky (Head of Neurology Department at the Technion and Head of Neurology at Rambam Hospital in Haifa). He suggested focusing on idiopathic pain (spontaneous pain causes of which are unknown),” he explains.
So the “initial idea was for an affordable digital device for pain relief in general, operated by a smartphone,” Ironi goes on, adding that “there are about seven diseases that are considered idiopathic and migraines stand out as the most prevalent.”
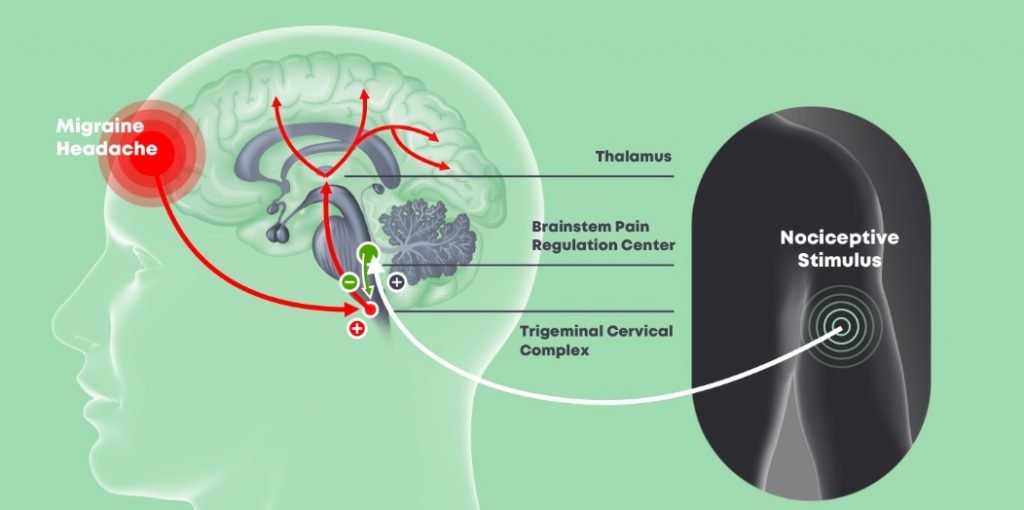
Ironi points out that the causes of migraines are unknown. “They are disruptions in neuro systems which start with sensations from hypersensitivity to light or sounds then the pain comes. In our bodies, there are natural systems that are supposed to take care of this type of pain by releasing neurotransmitters. This mechanism is descending pain inhibition mechanism [pathway]. A lot of times, this mechanism is not functional; the triggering doesn’t work, and several researchers found a high correlation between dysfunctional mechanisms and neurological diseases such as migraines.”
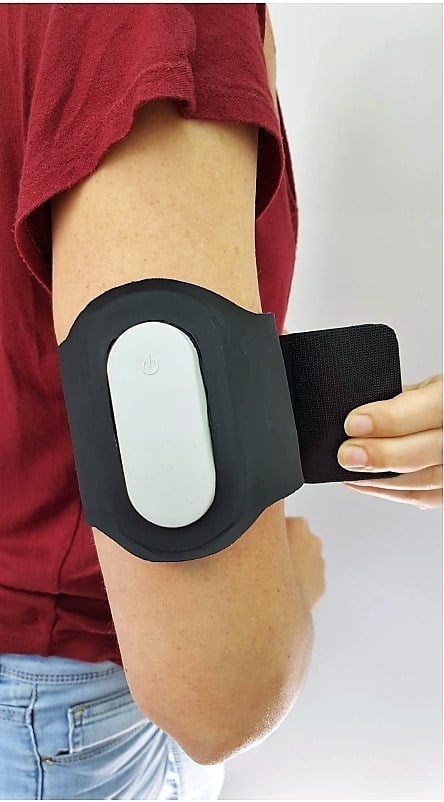
The Nerivio, which comes in the form of an armband, is abortive, not preventative. It uses electronic pulses sent from the accompanying smartphone app to stimulate conditioned modulation response to mitigate pain and keep track of migraine episodes. The app provides personalized treatment and helps the user keep track of previous headaches and treatment sessions.
It can be worn within 60 minutes of migraine onset.
Sign up for our free weekly newsletter
SubscribeFDA clearance
The device was cleared by the FDA for migraine relief in May 2019 after an extensive process that included research, pilot and pivotal clinical trials, and scientific studies which are still being published.
The Nerivio was given FDA clearance under the De Novo classification, meaning it is a novel device, which made its approval process different and slightly smoother as the company provided reasonable assurance of safety and effectiveness for the intended use. The authorization was based on the results of a randomized, double-blind, placebo-controlled, multi-center study where 252 patients from 12 clinics used the Nerivio to treat their migraine attacks. The findings were released in May 2019 in the peer-reviewed medical journal Headache: The Journal of Head and Face Pain, published by Wiley Periodicals on behalf of the American Headache Society.
Dr. Brian Grosberg, director of the Hartford Healthcare Headache Center in Connecticut who served as the lead Principal Investigator (PI) of the study said in a statement that the results “demonstrate a high efficacy ratio for single as well as multiple attacks, both at two and 48 hours after treatment.” Grosberg serves as a medical advisor to Theranica.
The language of the FDA approval also mentions the “lesser side effects” of the device, which gives it an advantage over prescription pain medications with their various and, sometimes extreme, side effects.
Ironi tells NoCamels that very mild side effects were found in less than 2 percent of the clinical trials and quickly disappeared shortly after treatment.
The Nerivio is priced at $99 and became available in select headache and migraine clinics throughout the US. Each device is good for 12 treatments of an average of 45 minutes, after which the device can be sent back to Theranica for responsible recycling, Ironi tells NoCamels.
The device will become available in Israel later this year, he says.
Not just migraines?
It appears Theranica won’t stop at migraines. Ironi previously indicated that the company has identified at least seven different painful conditions that may be relieved by non-invasive, drug-free technology “after appropriate clinical development.”
Besides headache disorders, idiopathic pain disorders include conditions as temporomandibular joint disorders (TMJD), fibromyalgia syndrome (FMS), irritable bowel syndrome (IBS), chronic pelvic pain, chronic tinnitus, whiplash-associated disorders, and vulvar vestibulitis (VVS).
“Based on the theory and medical advice, the device should be effective on other pain,” Ironi tells NoCamels. “The process to prove effectiveness on all other forms of pain is very long and complex. The studies done were for migraines specifically and that is what was submitted to the FDA. We plan to continue clinical development and move beyond migraines. Post-traumatic headaches are on the horizon for trial this year.”
To date, Theranica has raised $41 million from investors including aMoon, Israel’s largest healthcare VC, and Lightspeed Venture Partners. Dr. Shimon Eckhouse, a leading Israeli entrepreneur and investor in the field of medical devices and medical technology, serves as chairman of the board and is listed as a co-founder.
Related posts

Editors’ & Readers’ Choice: 10 Favorite NoCamels Articles

Forward Facing: What Does The Future Hold For Israeli High-Tech?

Impact Innovation: Israeli Startups That Could Shape Our Future




Facebook comments