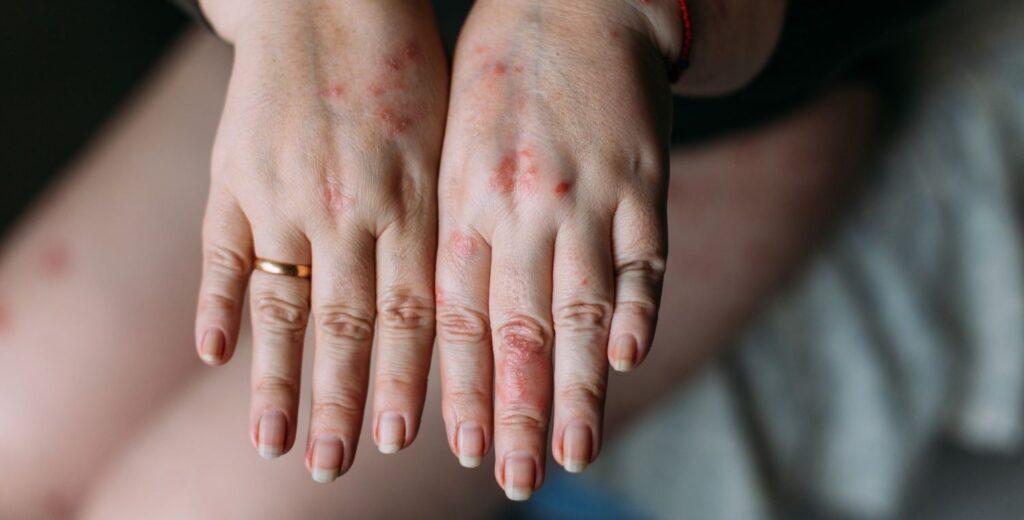
An Israeli biopharmaceutical company has announced successful preclinical results of new treatment for mild to moderate plaque psoriasis.
The antibody treatment, known as NanoAbs, was created by Jerusalem-based Scinai Immunotherapeutics, which focuses on the development of inflammation and immunology biological products.
Its creators compared the local injection to the way in which Botox is administered.
The treatment targets interleukin 17, commonly referred to as IL-17, a protein that promotes inflammation and plays a major role in the development of plaque psoriasis.
Presently, treatments for plaque psoriasis are primarily tailored to patients with moderate to severe symptoms, necessitating costly injections running into thousands of dollars.
Over half of psoriasis patients, those with mild symptoms, lack access to a safe and economical solution.
In trials, human skin models were injected with IL-17 to replicate the effect of plaque psoriasis symptoms. The models were then treated by two doses of NanoAbs, after which both noticeably improved and saw reduced psoriatic skin lesions.
“These positive results mark a significant step forward in the development of a novel treatment for the undertreated segment of mild and moderate plaque psoriatic patients,” said Dr. Tamar Ben-Yedidia, Scinai’s chief scientist.
“Scinai’s vision is to become the ‘Botox-like solution,’ providing a highly efficacious, specific, and safe biologic for local treatment of plaque psoriasis lesions.”
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments