A novel radiation treatment for cancer that targets tumors from the inside using alpha particles is now in Phase 3 pivotal trials ahead of receiving Food and Drug Administration (FDA) approval.
Jerusalem-based startup Alpha TAU is expanding its trials of the treatment for skin and other cancer, after its first trial of 10 patients succeeded beyond the company’s expectations.
The pilot trial focused on patients whose cancer was “unresectable [cannot be removed by surgery] or recurrent, who have no standard of care,” Alpha TAU CEO Uzi Sofer tells NoCamels.
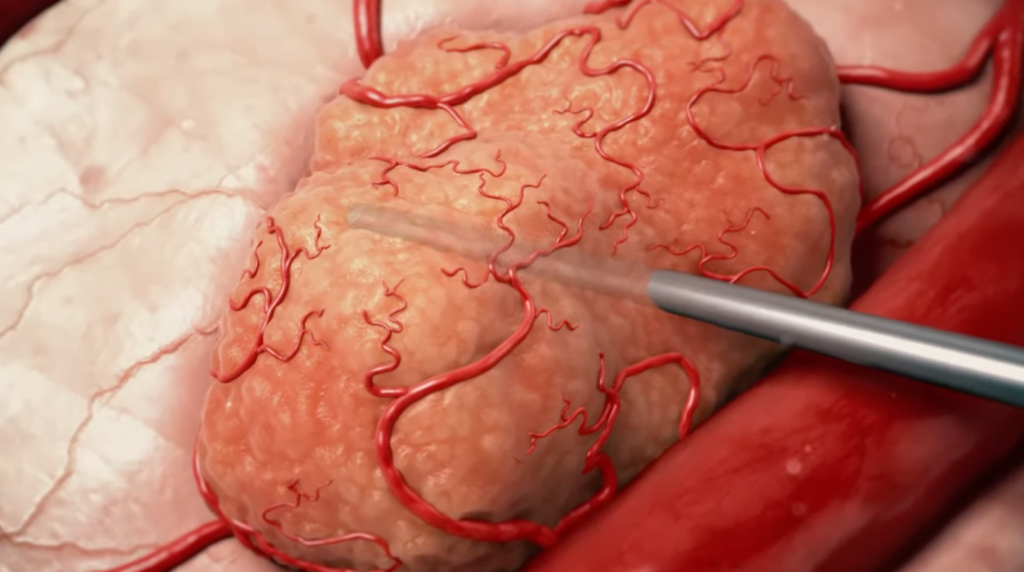
“Those patients got 100 percent CR [complete response],” Sofer says.
The pilot trial, conducted at multiple locations in the US last year, examined whether Alpha TAU’s DaRT (Diffusing Alpha-emitters Radiation Therapy) technology could successfully deliver targeted radiation therapy to patients with malignant skin and superficial soft tissue tumors that had returned or could not be removed surgically.
Alpha TAU had hoped that the treatment would be successful in at least seven of the 10 trial participants, but instead registered successful delivery to all 10. CT scans showed a 100 percent complete response rate at 12 weeks after the treatment and again at 24 weeks, with no evidence of the disease recurring in any of the subjects.
The trial also examined side effects from the radiation treatment, as well as the safety of the radiation, the stability of the device once placed inside the tumor and any other impact on the quality of life of the patient.
The results showed only mild or moderate side effects related to the device, and no systemic toxicity from it.
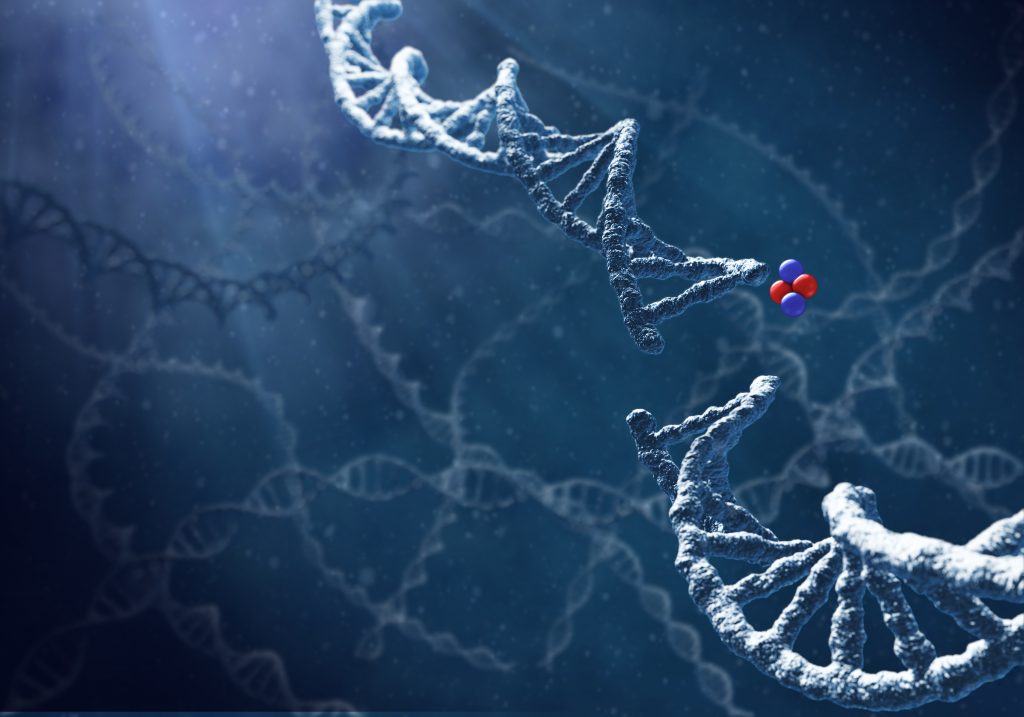
Radiation therapy for cancer normally uses beta and gamma particles. Alpha particles, while proving deadly for cancer cells in a tumor, are not traditionally used as they cannot travel far in solid masses.
Alpha DaRT, however, delivers the alpha particles directly into the tumor via a narrow device, inserted under local anesthetic, for a period of two to three weeks. The device is then removed and the patient monitored.
Because of the short range of the alpha particles, the treatment is focused on the tumor and not the healthy tissue that surrounds it.
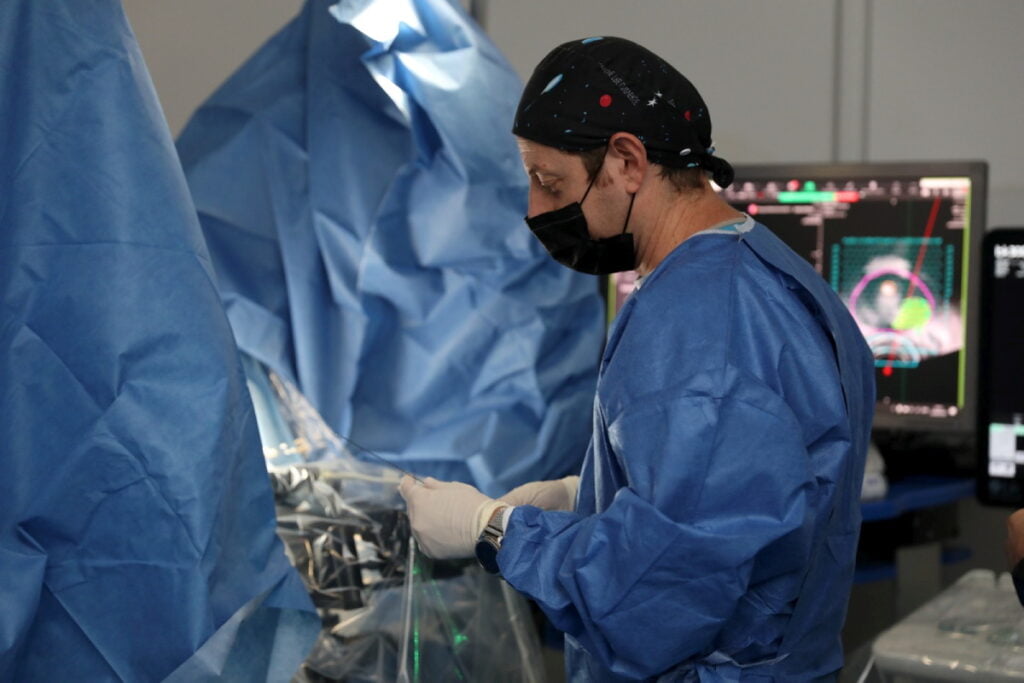
The findings of the pilot trial were published this month in the Journal of the American Medical Association (JAMA), months after submitting the results to the FDA.
The treatment is now undergoing its pivotal trial – the final one before the American agency gives it approval.
“We submitted the results that you see now to the FDA, and the FDA told us that we can submit the protocol for the last phase, the pivotal,” says Sofer.
Sign up for our free weekly newsletter
Subscribe
A pivotal trial is required by the US and European Union drug agencies in order to receive approval to market a new form of medication; studies can involve thousands of subjects and test the efficacy and impact of a drug.
Sofer says that the successful findings of the trial has led to medical institutes around the world clamoring to work with Alpha TAU, but for now research is limited to just a handful of locations for the pivotal trial.
“Many, many, many centers all around the world want to participate,” he says. “We are working with 20 centers in the US, two or three centers in Canada and another four in Israel that are going to participate in this trial.”
Sofer says Alpha TAU will be ready to submit the findings of the pivotal trial in around a year and a half from now.
“We will have six months of follow up, then we will analyze the results and send it to the FDA,” he says of the current trial. “The submission to the FDA can be in about 18 months from now.”
The revolutionary treatment is also being tried on other cancers, according to Sofer, who clarifies that, “right now it’s only for solid tumors.”
“We’re working on pancreas and lung and breast [cancer],” he says, explaining that the company is currently at various stages of testing for these other forms of malignant tumors.
“We have different indications [forms or symptoms of a disease] in different stages,” he says. “We treated the first pancreas patient two months ago, and we plan to treat a liver patient very soon.”

The device itself is easy to use and does not require specialized and often costly equipment in order to treat patients.
“When it is approved, it will be for any hospital, medical cancer center, all over the world,” Sofer explains.
“You don’t need any special equipment, and you don’t need the shielding,” he says, referring to the protective gear used in other forms of radiation therapy but are not needed for alpha particles.
“It will be very simple to implement. You don’t need special equipment or investment in capital expenditure or something like that, [just] regular tools.”
Related posts

Editors’ & Readers’ Choice: 10 Favorite NoCamels Articles

Forward Facing: What Does The Future Hold For Israeli High-Tech?

Impact Innovation: Israeli Startups That Could Shape Our Future



Facebook comments