Targeted therapies for cancer, which are designed to attack cancer cells without harming the normal cells around them, have revolutionized oncological treatments, improving efficacy and reducing side effects when compared to more traditional treatments.
But one side effect of targeted therapy – a prominent facial rash that resembles acne – has caused such an adverse impact that it is even deterring some patients from continuing their planned treatment regimen.
And this is an issue that Israeli startup EMRIS Pharma has tackled with a new carefully formulated ointment that patients apply onto their skin.
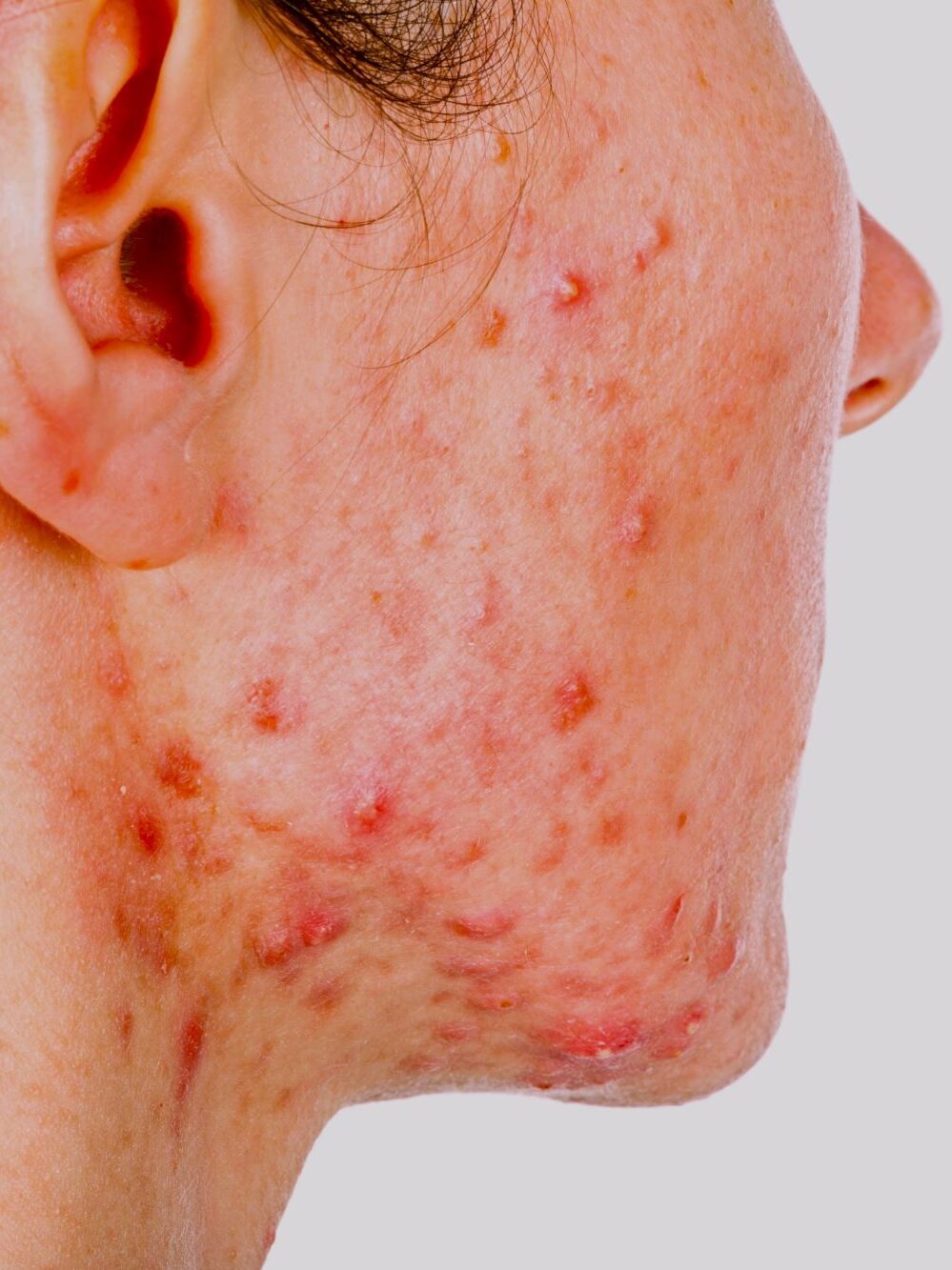
According to the American Cancer Society, a rash is the most common side effect of targeted therapies – its occurrence and severity is dependent on the type and the dose of the treatment. This rash, which resembles acne, usually appears on the face and in some cases other normally visible areas such as the neck, scalp and upper back.
And while the rash subsides once the treatment has been completed, it is so traumatic for some patients that they stop venturing into public or either reduce the frequency of their treatment regime or halt it altogether.
Dr. Sharon Merims, EMRIS CSO and co-founder, tells NoCamels that around 90 percent of patients receiving targeted therapies have some kind of skin toxicity.
Merims is a dermatologist who heads the Dermato-Oncology and skin toxicity clinic at the Sharett Institute of Oncology at Hadassah University Hospital-Ein Kerem in Jerusalem.
“The profile of the skin rash is very troublesome because it involves the face,” Merims says.
“[Patients] have a very unique facial rash, which is a very big problem for the quality of life. You can understand, once it’s on your face, you can’t really have a normal day to day.”
Merims began working on a solution eight years ago along with EMRIS co-founder and CTO Prof. Ofra Benny, a specialist in drug delivery systems at the Hebrew University of Jerusalem. The two mulled the possibilities of a topical solution to this issue – a cause of anguish for people already dealing with the implications of a cancer diagnosis.
What they were contemplating, she explains, was a targeted therapy to manage the side effects of a targeted therapy.
“I started thinking about an idea,” she says. “What if we can produce a blocking agent that we would apply topically, only on the skin, that would block the cancer drug from connecting to the receptor only at the toxicity site.”
During their research into the possibility of treating this rash locally, the two found the “potent lead molecule” that forms the basis of the EMRIS ointment that does – as Merims hoped – prevent the targeted therapy from affecting the skin cells.
The targeted cancer therapy itself is based on a substance that is an epidermal growth factor receptor (EGFR) inhibitor. This substance blocks the activity of the EGFR, which is related to cell growth and is found in many cells. By blocking EGFR, the treatment aims to prevent the cancer cells from growing, but also causes the skin side effect as the EGFR inhibitor attaches to EGFR on normal skin cells.

The company itself was set up in 2023 along with its third co-founder, Dr. Lyora Aharonov, an expert in biological research with a wealth of experience in R&D management. Funding came from the Israel Innovation Authority, the branch of the government dedicated to advancing the nation’s high-tech sector at home and on the international stage.
Sign up for our free weekly newsletter
SubscribeThe startup also received assistance from Yissum, the Hebrew University’s technology transfer company, which helped license EMRIS.
The EMRIS ointment, Aharonov tells NoCamels, blocks the EGFR inhibitor from binding to the skin cells and prevents the rash from developing, leaving the skin cells healthy and functioning normally.
“The key advantage of our treatment is its targeted action,” she says. “This is a real game changer for the patient.”
What makes this drug so unique, Merims explains, is that it is the only solution for the rash that deals with the root of the problem – preventing it, rather than merely trying to mitigate the symptoms.
“The accepted treatment today is avoiding the sun, putting on emollients and topical steroids with a regimen of antibiotics for two months” she says.
“That’s the protocol every patient is getting around the world. That’s the treatment for skin toxicity.”
Within the past year, Aharonov says, the company has very quickly reached “significant milestones,” primarily the formulation of the topical ointment that can specifically reach the necessary layer of the skin.
The formulated ointment has already undergone animal testing, with “very positive results for safety,” according to Aharonov.

Human trials are due to begin at Hadassah in early 2026. After that, the startup also plans to hold clinical trials in the United States, a necessary step for receiving approval from the US Food and Drug Administration.
The ointment is primarily designed for patients undergoing targeted therapy for advanced colon and head and neck cancers.
The priority, the company says, is keeping patients on their course of treatment for their cancer while improving the patients quality of life .
“Most patients, when they have had enough of the rash – and it comes pretty early on in treatment – will skip a dose or two, or get 50 percent of the dose or stop treatment altogether,” says Merims.
But the EMRIS solution, she says, “will help everybody on board these treatments.”
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss





Facebook comments