A new way of testing the eyesight of people suffering from diabetes lets these crucial yet all-too-often overlooked examinations be carried out with minimum discomfort and in a convenient setting.
People with both Type 1 and Type 2 diabetes are at very high risk of developing diabetic retinopathy, which is caused by damage to the blood vessels in the retina – the tissue at the back of the eye that captures light and translates it into sight – and can lead to blindness.
In fact, the World Health Organization, the UK’s National Health Service and the US Department of Health all agree that diabetic retinopathy is a leading cause of blindness in the developed world.
The only previous way to monitor diabetics’ retinal damage was with a regular check that involves dilating the pupil in order to flood the eye with light. And while a relatively short-lived experience, it is uncomfortable and incapacitating. Furthermore, it must be carried out in a clinical setting by a specialist eye doctor, adding to a long list of regular checkups that people suffering from the disease must carry out in addition to constantly monitoring their blood sugar levels.
But AEYE Health, a startup with headquarters in Tel Aviv and New York, developed an artificial intelligence-based method to test for diabetic retinopathy by a family doctor, at a pharmacy or even in a patient’s own home, with immediate results.
“The huge advantage in this exam is that it’s done in a fully non-invasive way, on the spot, no need to delay the patient,” AEYE Health CEO and co-founder Zack Dvey-Aharon tells NoCamels.
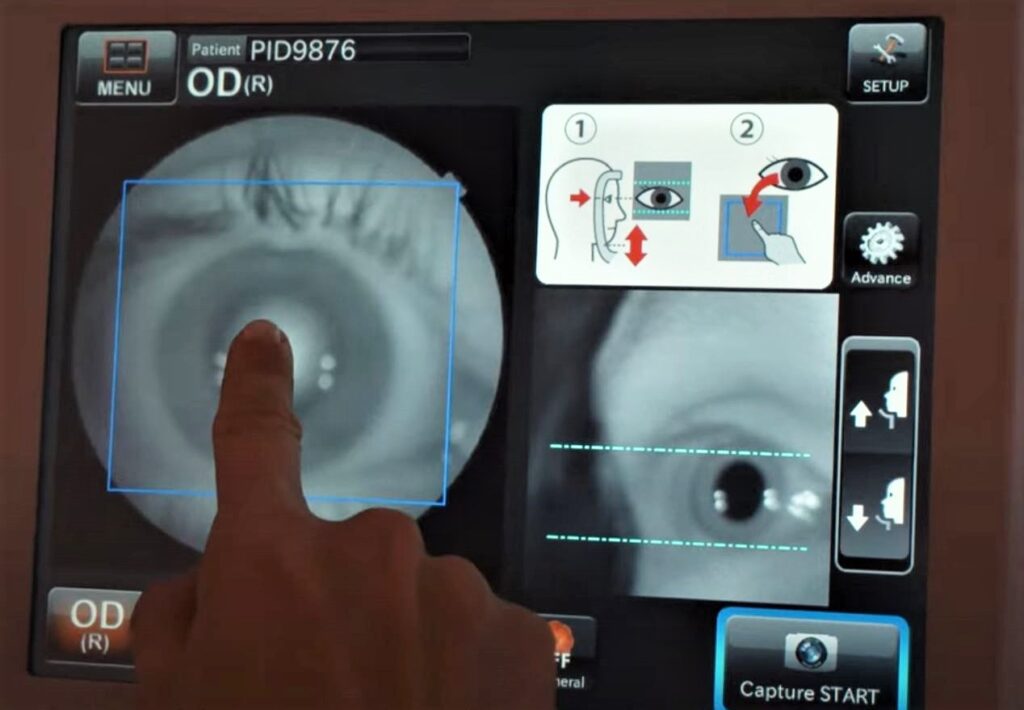
AEYE Health’s proprietary technology, currently only available in the United States, is AI-driven software that analyzes an image of the eye taken with a simple store-bought camera approved by the US Food and Drug Administration, and without having to dilate the pupil.
“We do not deal with hardware in any way,” Dvey-Aharon says. “The cameras are off the shelf, approved by the FDA [as is] our AI solution to do an autonomous interpretation of those images acquired by those cameras.”
In fact, the technology has received two separate authorizations from the FDA – initially for analyzing images captured by a tabletop camera and more recently for analyzing images taken by a mobile camera.
And because it is a small device, Dvey-Aharon points out that it is can be easily moved from one examination room to another, and then stored on a shelf or in a drawer.
This ease of access and testing is key to helping people actually keep eye doctor appointments, he explains. For while the annual check is crucial to preventing blindness in diabetics, the discomfort and inconvenience actually deter significant numbers from attending. This phenomenon of failing to attend necessary medical appointments is known as the “care gap.”
And, says Dvey-Aharon, the care gap when it comes to annual diabetic eye exams is considered one of the most important, because of how significant it is and how many people fall into it.
“All diabetic patients really need to do this exam on an annual basis,” he says.
“Unfortunately, most don’t actually follow through. They are aware they need to do it – they go to the person that treats their diabetes and they’re told to go and see the eye doctor. And they don’t go.”
Sign up for our free weekly newsletter
Subscribe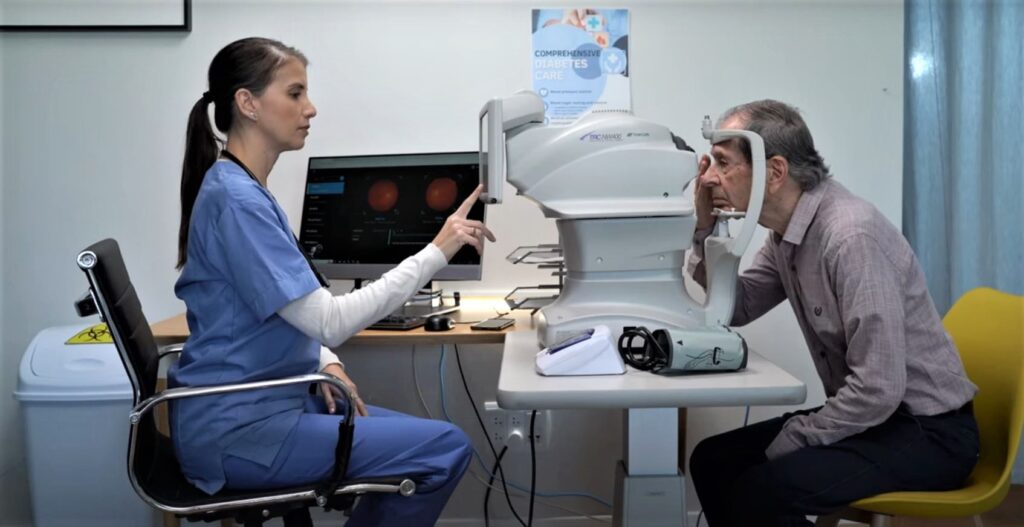
According to Dvey-Aharon, AEYE Health is the only company offering such a “clear solution” in the US, particularly now it has received FDA approval for mobile camera analysis.
Furthermore, he says, it is almost unheard of for the FDA to approve an AI diagnosis that is fully autonomous instead of being used as an accessory.
“Typically, the intersection of artificial intelligence and healthcare ends up as some sort of a decision support solution for physicians, some sort of measurement of something that physicians afterwards can use to determine,” he says.
“This is the only area where the FDA actually allows the AI to do the screening.”
The FDA approval came based on data in three clinical studies, which meant, Dvey-Aharon recalls, AEYE Health clearing “a super high bar.”
He explains that building the machine learning AI technology used in the platform was a lengthy undertaking that involved collecting and analyzing massive amounts of data in order to understand how to differentiate between patients whose eyes needed no immediate further care and those who required a referral to an ophthalmologist.
“There is no kind of a secret sauce, one element that is behind that, but actually a long, arduous process,” he says.
“If you want to really do things differently, there is a lot of innovation that needs to take place. And this innovation is in the end based on revelations, and the revelations are done during research processes.”
Founded in 2018 by Dvey-Aharon and COO Danny Margalit, the startup received vital funding from the Binational Industrial Research and Development (BIRD) Foundation, which encourages cooperation between US and Israeli tech companies.
Tal Kelem, BIRD’s Israel-based Director of Business Development, tells NoCamels that the fact that the foundation ended up investing more than planned in the startup indicates how important the organization believed the technology to be.
Dvey-Aharon in turn praises BIRD for creating a partnership between the startup and UMass Memorial Health, the largest health care system in Central Massachusetts, and along with it a collaboration with UMass Chan Medical School.
The FDA approval for the portable analysis platform has opened up new doors for the startup, which Dvey-Aharon says is about to announce a new collaboration with an American company that carries out millions visits to patients’ homes each year, in order to carry out blood tests or other forms of medical checks.
“Now, they will embrace this technology,” he says. “This is something you can actually do in the context of home health.”
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss





Facebook comments