Israeli startup SwiftDuct says the first clinical trial for its guidewire to ensure endoscopes enter the bile duct and not the adjacent pancreatic duct shows the potential to improve these procedures and their outcomes.
The bile duct is a tube-like structure that carries bile from the liver, where it is produced, to the small intestine, where it is used to help digest food.
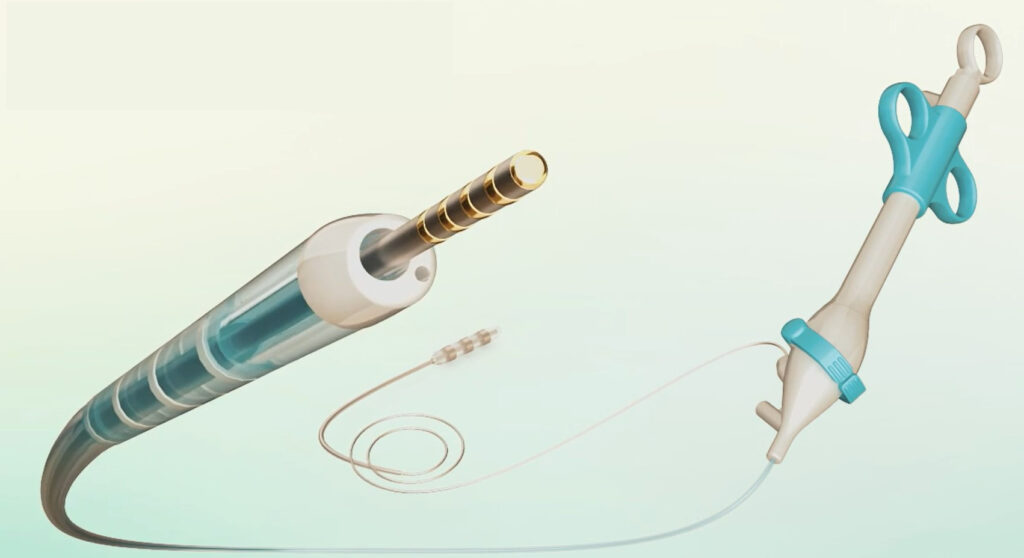
Any treatment of issues within the bile duct requires insertion of an endoscope – a tiny camera attached to a guidewire – during a procedure called endoscopic retrograde cholangiopancreatography (ERCP).
But the passage leading from the duodenum to the bile duct, known as the ampulla of Vater, also leads to the pancreatic duct.
Swift Duct has devised a method of differentiating between the fluids produced respectively in the bile and pancreatic ducts, which ensures that the endoscope enters the former and avoids the latter.
The trial involving 14 patients was carried out at Galilee Medical Center in Nahariya, northern Israel. It showed that the SwiftGlide device could successfully differentiate between the two fluids, the company says.
“We are thrilled with these initial clinical findings, though, of course, this is just the beginning and further studies should be conducted,” said Dr. Tawfik Khoury, the study’s principal investigator and Senior Consultant Gastroenterologist at Galilee Medical Center.
“SwiftDuct has in its hands a promising new technology that has the potential to enable endoscopists to quickly and efficiently conduct an ERCP procedure with selective biliary cannulation, improve patient outcomes and reduce the occurrence of post ERCP pancreatitis, hospital stays and costs,” said Dr. Wisam Sbeit, Head Manager of the Gastroenterology Department at Galilee Medical Center.
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments