Israeli biopharmaceutical company BioLineRX, which develops therapies for cancer and rare diseases, has signed an agreement to conduct Phase 1 clinical trials of a potential treatment for sickle cell disease (SCD), at multiple locations in the US.
The trial is sponsored by St. Jude Children’s Research Hospital in Tennessee, which has affiliates across the country and also involves Washington University School of Medicine in St. Louis.
The study will evaluate the impact of the drug motixafortide, which is currently used to treat a form of blood cancer, in mobilizing stem cells in developing gene therapies for SCD.
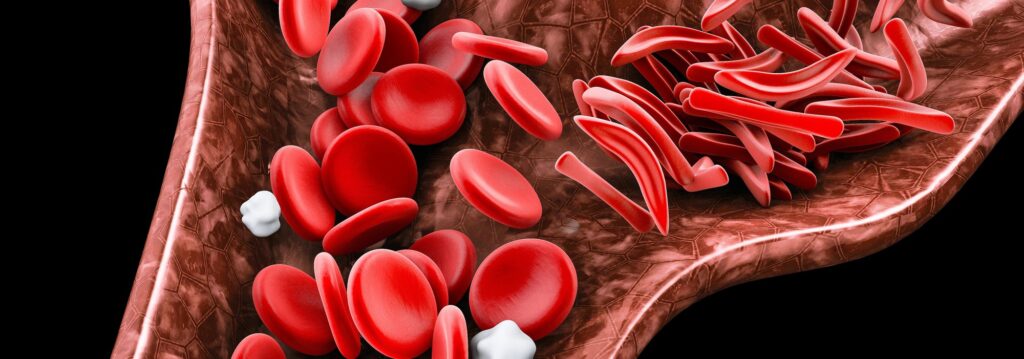
One of the world’s most common genetic diseases, SCD affects some 20 million people across the globe, including more than 100,000 people in the US alone. It disproportionately affects people of color.
The disease is caused by mutations of the hemoglobin gene, which contains instructions for making a protein found inside red blood cells, and causes those blood cells to take on the abnormal shape that gives SCD its name. Symptoms of SCD include anemia, blocked blood vessels and lack of blood flow to multiple organs.
“The recent FDA approvals of two gene therapies for sickle cell disease in the US is an exciting development for the sickle cell community,” said Ella Sorani, PhD, Chief Development Officer at BioLineRx.
“Through this new trial with St. Jude, and our ongoing collaboration with investigators at Washington University School of Medicine in St. Louis, we are excited to be working with leaders in gene therapy and stem cell mobilization to evaluate potential new mobilization options for patients with SCD,” she said.
Enrollment in the study, which will involve 12 patients aged 18 and above, is set to begin in the coming months, with initial data expected in the second half of 2024.
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments