A new treatment for early-stage Alzheimer’s uses nanoparticles and light to slow the progress of the disease, all set off by a simple, low-powered x-ray.
And according to the Israeli university where it was developed, this innovative therapy has shown delayed progression of the disease by up to 36 percent in patients with early-to-mild cognitive impairment.
The treatment was developed by a researcher at Bar-Ilan University near Tel Aviv in conjunction with scientists in Italy, and takes a different approach to an accepted method of slowing the spread of Alzhiemer’s.
The disease, which according to the World Health Organization is the most common type of dementia, affecting up to 40 million sufferers worldwide in 2023, is known to have no cure. And even though it was first diagnosed in 1906, there has yet to be a universally accepted cause of the disease.
The Amyloid beta (A-beta) protein is believed to be one of the primary factors in the development of Alzheimer’s, as it forms what is known as amyloid plaque that accumulates in the brains of people with the disease. This plaque forms in the spaces between the nerve cells, which disrupts cell function in memory, atrophying key areas of the brain and ultimately causing significant loss of its functionality.
The existing treatments that focus on A-beta center on a non-soluble form of the protein as the cause of the plaque, while the novel treatment developed under the auspices of Prof. Shai Rahimipour of Bar-Ilan’s Department of Chemistry attacks the soluble, oligomeric (made up of repeating units of the same limited number of molecules) form.
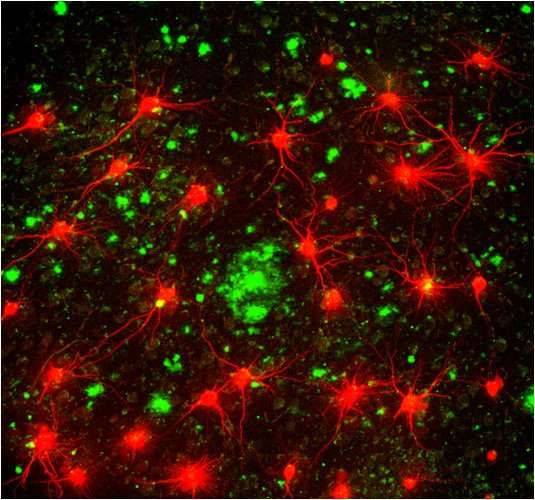
New research pointed to plaque formed by this smaller, soluble form of the A-beta protein as causing the neural impairment and cognitive decline of Alzheimer’s rather than the long-targeted non-soluble form.
“Until 10 years ago, it was a consensus that these non-soluble networks of plaques are responsible for Alzheimer’s,” Rahimipour tells NoCamels.
“For that reason, many companies started to develop diagnostics and therapeutics to treat these non-soluble amyloids,” he says.
But, he explains, while they were able to reduce the amount of the non-soluble amyloids, this did not have any real impact on the progression of the disease, and people were still dying from it.
“So it became a question of whether these non-soluble amyloids are responsible for the disease,” he says.
Based on this new research, Rahimipour together with Professors Angelo Monguzzi and Marcello Campione from the University of Milano-Bicocca decided to target the smaller, soluble molecules rather than the larger, non-soluble ones.
The three researchers designed nanoparticles that would target the early stages of these soluble A-beta proteins, preventing them from clumping (aggregating) inside the brain and forming the amyloid plaque.
Rahimipour explains that he has been considering this avenue of treatment since he opened his lab a little over a decade ago.
“We suspected from the beginning that this oligomeric form is more toxic, because you can see the same phenomena of toxicity with small proteins, which when they are aggregated in the soluble form are very, very toxic,” he says.
Sign up for our free weekly newsletter
Subscribe“So we started to generate molecules that can specifically bind to this kind of oligomer and inhibit the toxicity and aggregation of the plaques.”

The scientists drew inspiration from cancer research, specifically the concept of using light to inhibit harmful proteins.
They decided to use molecules that have a chemical reaction to light (photosynthesizers), which could harm A-beta’s amino acids – the molecules that make up all proteins.
To do this, they used a photosynthesizer that has already been approved by the US Food and Drug Agency (FDA).
“We showed very nicely that when you are incubating A-beta with an FDA-approved photosynthesizer, you can damage A-beta very specifically in very specific amino acids and inhibit the aggregation [of] these oligomers – and we saw that the toxicity is going down,” he says.
They dealt with the issue of triggering the reaction to light inside the body when they stumbled across research showing certain nanoparticles can create a light source when exposed to x-rays.
“Basically it means that if you have these kinds of nanoparticles and you shine an x-ray, it generates visible light,” he explains. “And this visible light can activate our photosynthesizer.”
So the treatment became a two-stage therapy: First, they introduced the photosynthesizer to the A-beta protein in the brain. Then, they introduced the nanoparticles that when x-rayed trigger the light reaction by the photosynthesizer needed to impact the amino acids. The x-rays are very low powered in order to shield the brain as much as possible from harm.
The researchers tested their hypothesis in very small worms, reproducing the soluble A-beta molecules and then the effect of the nanoparticles and photosynthesizer on them.
They are now about to embark on the proof-of-concept stage using larger animals and hope to have more solid results in the next six months, although Rahimipour warns that actually bringing the therapy to market is still several years away.
Although still in the preliminary stages of development, the scientists are now looking to partner with an established pharmaceutical company for funding and support to help navigate the long path to commercialization.
“We have a lot of experience with early diagnosis of Alzheimer’s disease,” Ranimipour says.
“The most important thing is the notion that Alzheimer’s disease should be detected in its very early stage and this is what we have in mind.”
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss





Facebook comments