An Israeli medtech company whose novel therapy freezes and destroys malignant tumors has announced positive results in a trial of breast cancer patients.
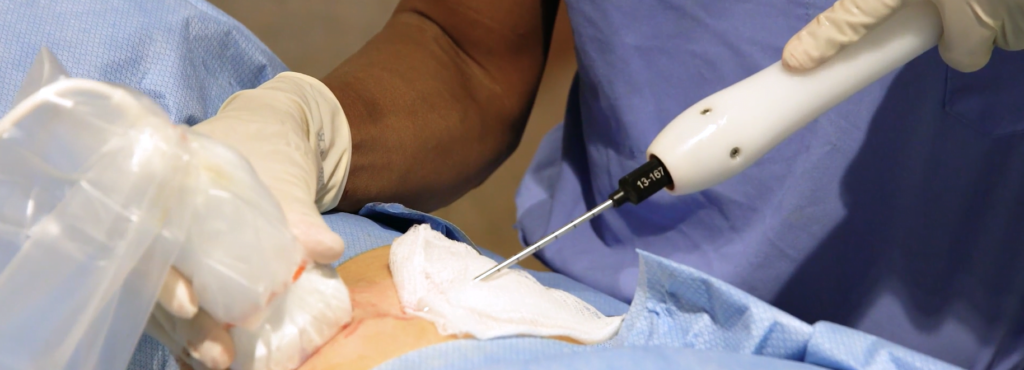
IceCure Medical’s ProSense system uses extremely cold liquid (cryoablation) to freeze and destroy abnormal tumors as an alternative to surgical removal. Its focus is currently on early-stage breast, lung, liver and kidney tumors.
While cryoablation is not a new process, IceCure Medical’s system allows physicians to perform the procedure in their own offices, with no need for hospitalization.
The company’s ICE3 study is the largest multicenter clinical trial performing cryoablation on low-risk and early-stage breast cancer tumors.
The results of the trial were released after a five-year follow up to monitor the health of the people who participated in it. Of the 194 patients treated, IceCure reported, 187 had no signs of local recurrence of the tumors or other significant complications.
Based on these results the company plans to submit a request to the US Food and Drugs Administration for permission to market the product in the United States. The company says it currently has approvals in Europe and China.
“We are very pleased with this topline outcome and believe these results demonstrate a highly favorable safety and efficacy profile that positions ProSense as a desirable alternative to lumpectomy for early-stage breast cancer,” said IceCure CEO Eyal Shamir.
“While the FDA evaluates the data, we are optimistic their upcoming decision will give women in the US the same access as those who are already benefitting from ProSense in other countries,” he said.
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments