An Israeli company has been granted clearance from the US Food and Drug Administration (FDA) for its software used in the removal of liver tumors.
The BioTracelO software created by Rehovot-based TechsoMed Medical Technologies received De Novo clearance from the FDA, which is approval granted to a novel system.
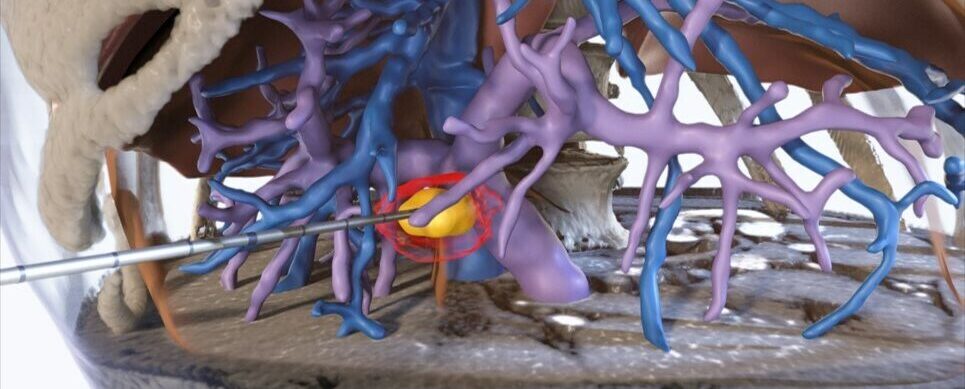
The software is used during ablation therapy – a minimally invasive treatment that uses extreme temperatures to kill tumors – and is designed to predict the tissue response to the therapy.
It is also designed to aid physicians who are performing liver tumor removal by providing real-time visuals with the ultrasound imaging.
During the procedure, the software offers a visual display of the ablation zone, correlating it with imaging from a CT scan taken 24 hours beforehand in order to observe any rapid changes in the area. It also predicts how the area will look in another 24 hours.
The system has been validated in a multi-center study in the US that involved 50 patients.
“Techsomed has taken a major step towards making our vision for image-guided ablation therapy a reality,” said TechsoMed’s CEO and founder, Yossi Abu.
“We are genuinely excited to be able to offer physicians smart solutions that bring a new level of precision to ablation therapy.”
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024
TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments