A new handheld, self-scanning ultrasound device that attaches to a smartphone allows pregnant women to see their baby in the womb at any time and in the comfort of their own home, without a visit to a hospital or clinic.
According to Pulsenmore, the startup that makes the device, one of the main reasons why pregnant women go to the emergency room is because they cannot feel any movement of the fetus.
Women in Israel are able to get the devices through their healthcare providers, the largest of which – Clalit – has worked closely with Pulsenmore.
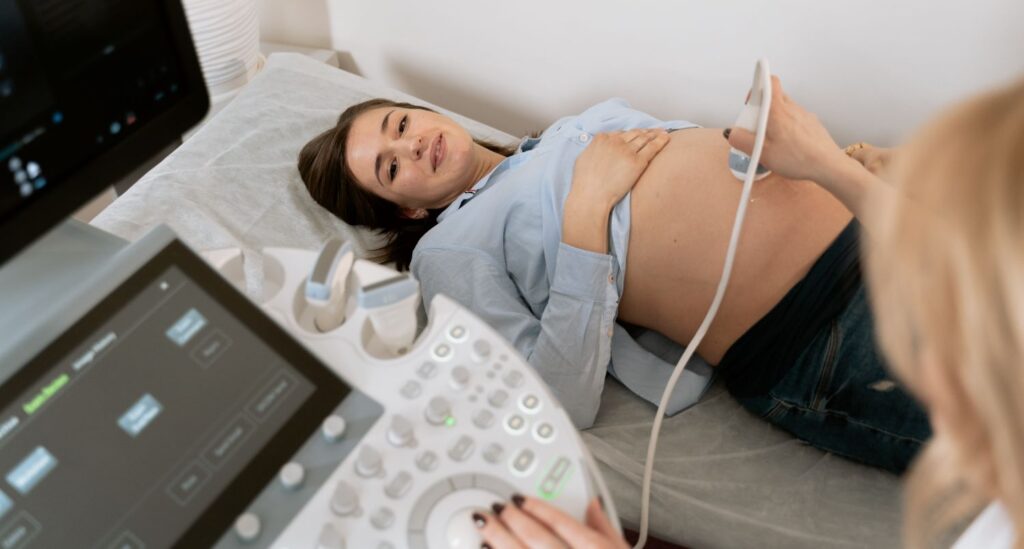
The device consists of a compact handheld unit equipped with advanced ultrasound technology that is attached to the user’s phone, allowing a woman to see her baby on the screen and send the images to her physician.
The dedicated mobile application accompanying the device provides a user-friendly interface via a smartphone or tablet, allowing expectant mothers to visualize the ultrasound images, track fetal movements and listen to the baby’s heartbeat with ease.
The device is the initiative of Pulsenmore CEO and founder Dr. Elazar Sonnenschein. He was inspired to create the handheld device for mothers-to-be to perform scans at home and transfer them to their physicians following a pregnant relative’s health scare.
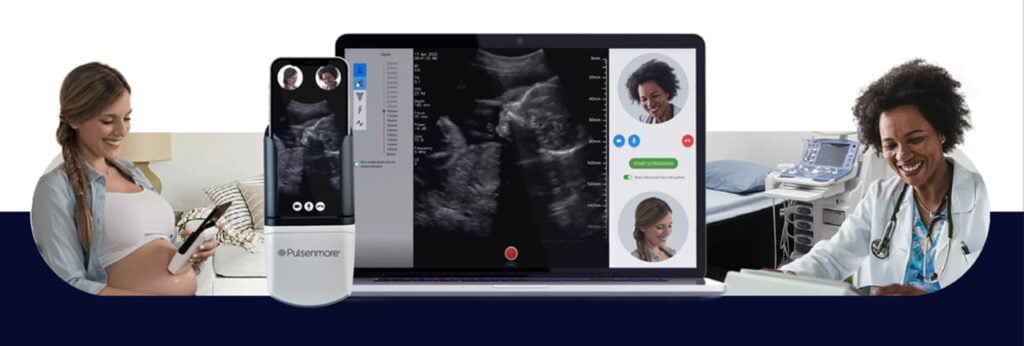
The key feature of the device, Sonnenschein explains, is that the data from the scan can be delivered directly to a medical team or for review via the internet, potentially negating the need for a pregnant woman to spend hours at the ER.
“The most important thing for us is the transmission. Each mobile phone can transmit the information packet,” he tells NoCamels. “We transmit it to the cloud and the reader can see it. The reader can be a sonographer, a physician or midwife or nurse who can review it in the cloud immediately.”
The scanner aims “to provide the women with a simple tool to scan the belly, so the healthcare professional can review and immediately see the parameters,” Sonnenschein says.
“For years, there was a perception that ultrasounds can only be conducted by professionals. In general, it’s true, but this is only in general. There is room for simple applications in ultrasound that allow a user to do the [procedure].”
These waves are then transformed into real-time images and audio representations of the baby’s heartbeat and movements.

To initiate the scanning process, the pregnant woman gently places the device on her abdomen. The device then emits harmless ultrasound waves that penetrate the body, allowing for a non-invasive examination of the fetus.
“We can see the heartbeat, we can see movement, we can see amniotic fluid volume, we can see the placenta location,” explains Sonnenschein.
The device can be used from the 14th week of the pregnancy, but women in the third trimester (from week 27 onwards) will have a clearer image of their baby.
“After 27 weeks gestation, we can briefly see the fetal tone,” Sonneschein says, referring to the baby extending or flexing a limb or a hand in the womb.
“These are the important parameters that provide a good understanding of the vital signs of the fetus. I need to point out that we are not looking for defects. We are not looking to see if there are issues with the fetus’ heart. For that purpose, the pregnant woman needs to go for a very professional scan.”
The partnership between Clalit and Pulsenmore has paved the way for groundbreaking collaboration in the field of remote healthcare.
“They did more than 65,000 scans, which is a lot. There are more than 6,000 women using the device,” says Sonnenschein of the agreement with Clalit.
Pulsenmore offers two modes of healthcare support for users of the ultrasound.
Sign up for our free weekly newsletter
SubscribeThe first mode allows one-on-one meetings through a telehealth service, where expectant mothers perform the ultrasound in real time and receive immediate feedback and guidance.
The second mode enables women to carry out the scan at home with app guidance and upload the data to a central database for interpretation. Medical professionals later analyze the scans and provide results and recommendations.
This second option is the one currently in use in Israel, with trained staff responding swiftly to any perceived problems.

“We have had cases in which a woman uploaded the image and within one hour the ambulance was taking her to the hospital because she suffered from low amniotic fluid levels,” explains Dan Gus, Pulsenmore’s director of capital market and operations.
The start of COVID-19 had a significant impact on Pulsenmore’s production, as it “expedited everything,” Sonnenschein says. It also highlighted the critical role the ultrasound devices played in providing remote healthcare solutions during a challenging period.
With strict rules about contact with other people in place globally, the company quickly delivered their devices to HMOs to distribute to pregnant women who had been advised to avoid hospitals.

Pulsenmore has received support from the Israel Innovation Authority, a government body promoting research and development in the country, securing 1.7 million shekels to advance its work.
Clalit is also working with Pulsenmore within the context of IVF treatments.
The healthcare provider aims to offer its patients undergoing IVF the ability to perform ultrasound scans at home, using a different device, so that they can keep track of their fertility without frequent hospital visits.
“It enables women who are offered IVF to measure the follicles [egg sacs within the ovaries] at home, so they are not required to attend the hospital two, three, or four times a week. They can do the measurements from home and the healthcare professional can measure the follicles and advise the woman if she needs to attend the department in order to harvest the egg,” says Sonnenschein.
Pulsenmore also plans to be able to scan for renal disease and congestive heart failure.

The startup has entered into a partnership with American multinational medical technology company GE Healthcare, which is investing up to $50 million in Pulsenmore and the distribution of its products in major markets around the world.
“We are happy to do business with GE, they are number one in the market. They are also very professional and now they’re distributing the device in Europe,” says Sonnenschein.
While other companies around the world also produce portable ultrasounds, Gus says only its product can be used by women at home without supervision from a medical expert.
“That’s our uniqueness,” he says.
Sonnenschein tells NoCamels that the company has big plans for international expansion in the coming year.
“Hopefully, in a few months, we will gain the [FDA] clearance in the US and they will be able to start sales and marketing. We are also working in Japan to get clearance,” he says, adding that they also want to expand to Australia, South America and more.
“We will continue to bring new devices and applications for home users and to eliminate visits to the hospital. This is our mission. We are very focused, we are very enthusiastic.”
Related posts

Editors’ & Readers’ Choice: 10 Favorite NoCamels Articles

Forward Facing: What Does The Future Hold For Israeli High-Tech?

Impact Innovation: Israeli Startups That Could Shape Our Future




Facebook comments