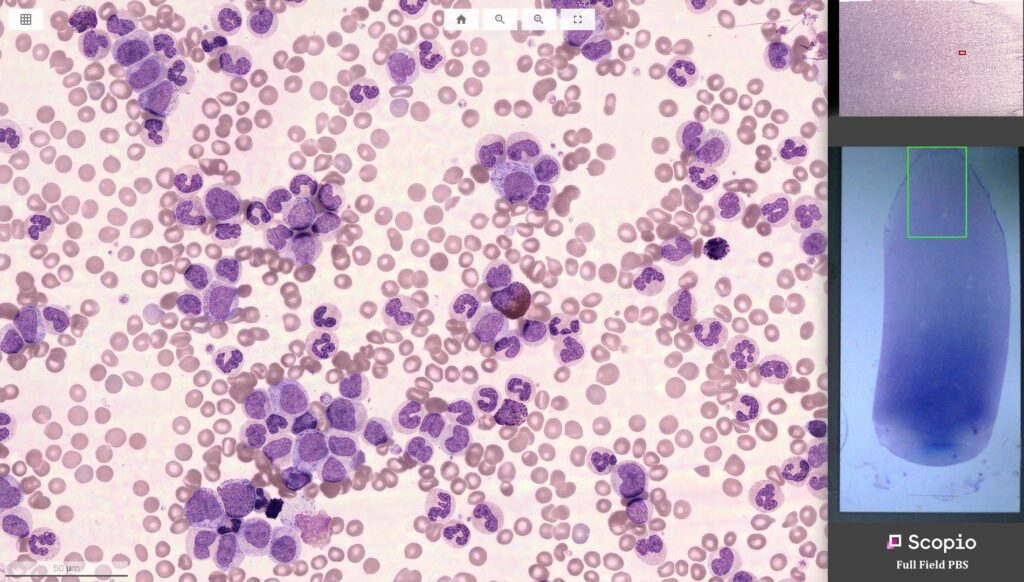
Israeli digital microscopy company Scopio Labs, announced this week that it has received US FDA 510(k) clearance for its X100HT device with Full-Field Peripheral Blood Smear (PBS) Application to improve the accuracy and speed of hematological tests.
As hematological disorders such as blood related cancers, anemia, infections, and allergies continue to grow more prevalent around the world, peripheral blood smear reviews have risen to become crucially important diagnostic tools for accurate analysis. Most of these tests are in most casts performed manually, however, Scopio’s Full-Field PBS morphology solution eliminates the need for additional manual microscopic examination entirely.
Scopio’s devices are capable of capturing large scan areas at 100X magnification, as opposed to traditional manual microscopy which are unable to provide high resolution and large field views simultaneously. The X100HT can hold 30 slides and process up to 40 samples per hour, and is therefore well suited to meet the high throughput requirements of large hospitals and labs.
Founded in 2015 by Itai Hayut and Erez Na’aman, Scopio Labs’ platform automates the imaging of full microscopy samples into unique high-resolution digital scans using state-of-the-art computation photography techniques. The company has also built and integrated various AI and remote consultation solutions for large and small labs and hospitals to improve diagnostic processes and reduce turnaround time. The company has raised $73 million dollars to date from investors such as OurCrowd, Aurum Ventures, and Ilex Medical, among others.
“We’re excited to expand our suite of fully digital AI-powered diagnostic platforms to accelerate PBS analysis, improve consistency of results, and reduce review time,” said Erez Naaman, CTO and co-founder of Scopio Labs. “At Scopio, we are determined to usher in the digital revolution to laboratory medicine. Our devices offer complete remote capabilities for real-time diagnosis and treatment decisions, allowing experts to review, collaborate, and consult from anywhere, and at any time, using our AI-powered applications.”
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments