Israeli medical startup Salignostics, a company specializing in saliva research and tech development, announced on Thursday that it will start commercializing Salistick, the world’s first and only saliva-based rapid pregnancy test kit.
This rapid pregnancy detection kit is a highly accurate and cost-effective tool that leverages saliva-based hormone detection technology pioneered by Salignostics and delivers accurate results in minutes. The kit is also safe, hygenic, and non-invasive, the company said in an announcement.
The commercialization of SaliStick is set to begin early next year
Saliva contains over 5,000 identified proteins that mirror and overlap the physiological state of one’s blood, according to Salignostics. Drawing from the same principles used to develop SaliCov, its highly successful rapid antigen saliva test kit to detect COVID-19, Salistick can detect the pregnancy hormone β-hCG in saliva and delivers proven and accurate detection rates for early pregnancy
Salignostics said it is in very advanced stages of receiving the European Union’s CE Mark for Salistick and has completed a 510(K) initial Q-submission of the test kit for the FDA in the US. The company has also successfully completed clinical trials in Israel on more than 300 women, both pregnant and non-pregnant, and has completed thousands of analytical trials.
“Saliva is the key to rapid diagnostics for a variety of medical reasons. Quintessentially it is the only non-invasive, easy, and hygienic means to detect hormones, viruses, and even diseases,” said Dr. Guy Krief, co-founder, Deputy CEO, and Director of Business Development, Salignostics. “With Salistick, we are leveraging the powerful diagnostics abilities we have been able to create from analyzing saliva. We are delivering a product that completely removes the need for blood and urine samples when testing for pregnancy.”
Salignostics was founded in 2016 by a group of five like-minded PhDs who identified a growing need for a cost-effective means to monitor health status, disease onset, progression, and treatment outcome through non-invasive approaches that are convenient, simple to use, fast and accurate. Their research led them to unlock the power of saliva as it contains many of the same analytes as plasma and is, in many instances, a mirror of the physiological states of blood. The pioneers of this technology include Professor Aaron Palmon, Salignostics President and former Dean of the Faculty of Dental Medicine at the Hebrew University, Omer Deutsch, CEO, Yoav Neumann, Senior Researcher, Raluca Cohen, Chief Scientific Officer, and Guy Krief, Deputy CEO and Director of Business Development.
Sign up for our free weekly newsletter
SubscribeThe annual global market for pregnancy tests is estimated at over $2 billion, with an estimated hundreds of millions of tests sold every year. However, these tests all rely on results derived from blood or urine samples and often need to be conducted by a medical professional. Salistick can be used anywhere, at any time of the day; it is clean, painless, and can detect the β-hCG pregnancy hormone safely on the first day of a missed period without the intervention of medical staff.
Research conducted by Salignostics shows that more than 68 percent of women surveyed would instead opt for a saliva-based test kit over traditional pregnancy detection tests, while 21 percnet said they had no preference. A primary reason cited for this is the overall user experience, convenience, and cleanliness of the product.
A pioneer in saliva-based testing and diagnostics, the company’s SaliCov simple COVID-19 antigen saliva test kit has proven highly effective and received a CE Mark from the European Union for marketing throughout Europe. To date, the company has provided hundreds of thousands of kits to Europe and Africa, and the test kits are the only saliva-based rapid antigen COVID tests that participate in the U.S. National Institute of Health’s (NIH) RADx accelerator program.
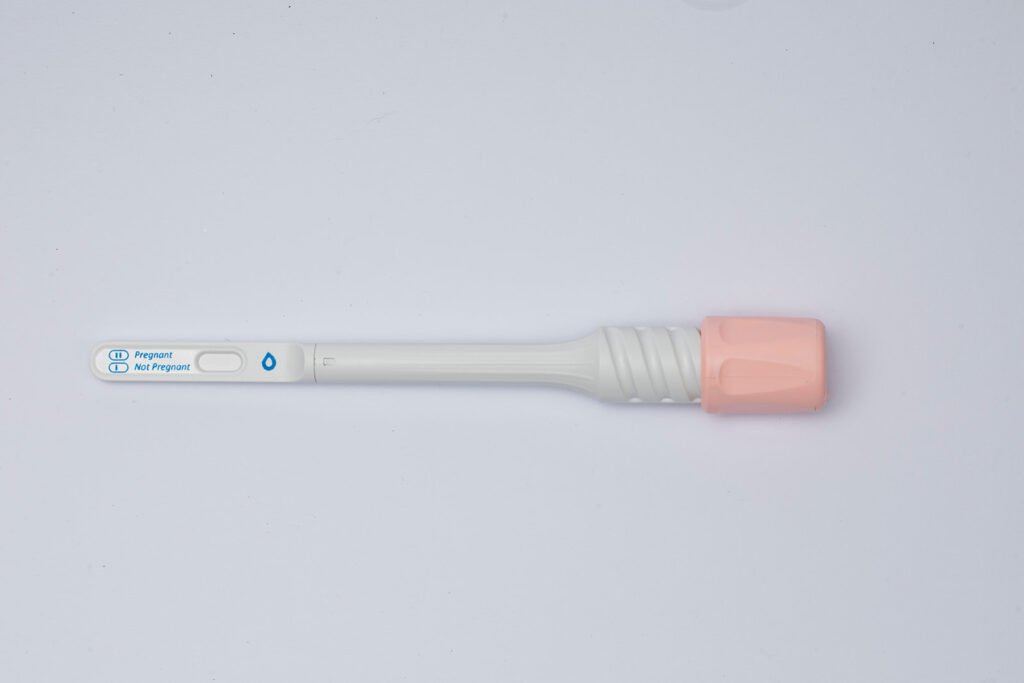
Like the SaliCov kit, Salistick comes individually wrapped for hygienic purposes and has a handheld applicator for collecting saliva. Once the sample is collected (about one minute), the applicator is replaced via an intuitive twist and click mechanism into the compact analyzing unit while the results are analyzed. These results are then displayed on a results window, where two lines indicate a positive test, and one line means negative.
Results are derived exclusively on saliva analysis and are based on a platform technology. As the technology is not biomarker specific, it can be replicated and used for multiple applications and use cases—for example, COVID-19 and even malaria detection.
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments