Israeli AI-powered cancer diagnostics company Ibex Medical Analytics announced this week that it has launched a new system to detect gastrointestinal cancer.
The company will deploy the system at KSM (Kahn-Sagol-Maccabi), the research and innovation institute of Israel’s second-largest HMO Maccabi Healthcare Services.
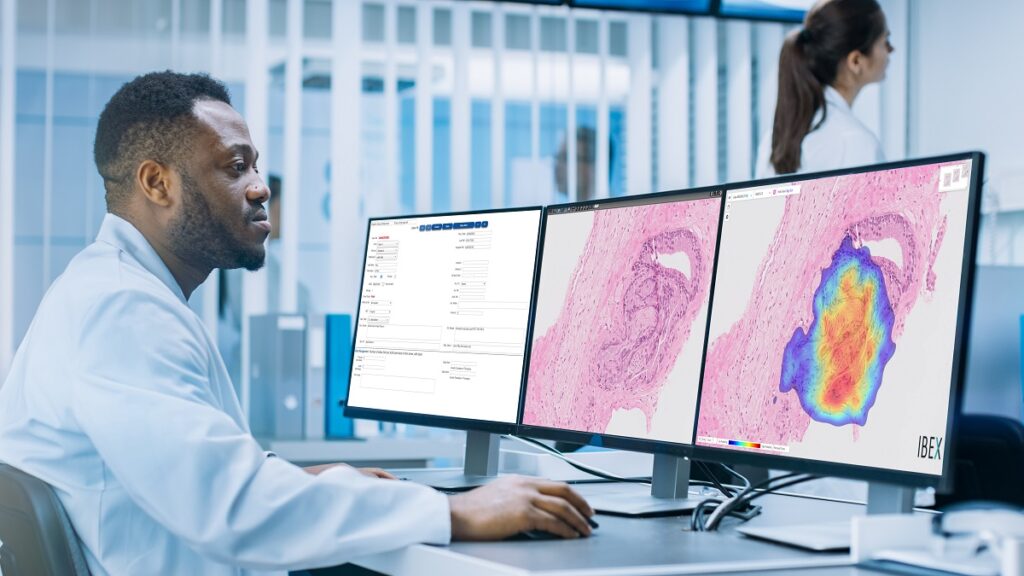
Ibex’s Galen software is the first AI-powered cancer diagnostics solution in routine clinical use in pathology and is deployed in labs worldwide. These solutions empower pathologists to improve diagnostic accuracy and enable a more efficient workflow, Ibex has said.
In June, the platform received a Breakthrough Device Designation from the United States Food and Drug Administration (FDA). The FDA’s Breakthrough Device Designation is granted to technologies that have the potential to provide more effective treatment or diagnosis of life-threatening diseases, such as cancer. Ibex says the designation will enable close collaboration with, and expedited review by the FDA, and provides formal acknowledgment of the Galen platform’s potential benefit as well as the robustness of Ibex’s clinical program.
Galen Gastric is an integrated diagnostics and quality control solution that supports pathologists in the detection of gastric cancer, dysplasia, H. pylori, and other important clinical findings. The solution is an addition to Galen Prostate and Galen Breast that have already been deployed at Maccabi Healthcare Services, as well as other labs worldwide, where they are used in everyday clinical practice.
With this deployment, Maccabi becomes the only health system in the world to use AI for multi-tissue cancer detection on breast, prostate and gastric biopsies, supporting their pathologists with improved accuracy, quality control and efficiency, Ibex said in a statement.
Gastric cancer is among the most common malignant diseases in both men and women worldwide, with over a million new cases each year. While pathologists play a crucial role in the detection and diagnosis of cancer, a rise in cancer prevalence and advances in personalized medicine have resulted in growing diagnostic complexity.
“Galen Gastric demonstrates that our Strong AI is the leading approach for empowering pathologists with solutions that support their real-world needs,” said Dr. Chaim Linhart, co-founder and CTO of Ibex. “The Galen platform, recently granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA), lays the foundation to clinical-grade computational solutions that accurately identify dozens of features and become the physician’s perfect companion, helping provide every patient with an accurate, timely and personalized cancer diagnosis.”
Scans for gastric cancer examined by pathologists will also be reviewed by the Ibex Second Read system. The Galen platform offers the Ibex First Read and the Ibex Second Read, applications that analyze cases prior to human pathologist review, enabling case prioritization, and in parallel with human pathologist review to identify any discrepancies, respectively. The Second Read system for breast and prostate biopsies have been used at the Pathology Institute of Maccabi Health Services – Israel’s leading HMO – since 2018 and the First Read implementation was announced late last year.
“We are excited to add Galen Gastric to what is the most comprehensive AI deployment in pathology, supporting us in providing quality diagnosis to our patients,” said Judith Sandbank, MD, Director of the Pathology Institute at Maccabi Healthcare Services. “The clinical benefits from using Ibex’s AI solutions have been the key driver in Maccabi’s decision to fully adopt digital pathology, and we are impressed by how fast AI technology has become an indispensable part of our diagnostic pathway. We look forward to benefiting from its new insights in everyday practice.”
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments