Israeli biomedical company IceCure Medical, the developer of technology that freezes cancerous tumors, announced that it raised NIS 20.7 million ($6 million) in an equity share offering to investors last week.
The company said the capital raising was supported by Rosario Capital, Yair Capital Offerings and Dinance Ltd., Apax Underwriters and Offerings, offering consultant Yaron Iluz and Naor El-Hai and Advs. Reut Alfiah, Oded Har Even, and Gal Cohen from the Sullivan international law firm.
It also reported that the offering was oversubscribed with a demand of NIS 24 million ($7 million.) In other words, investors ordered more shares than were being issued.
IceCure CEO Eyal Shamir says the offering “ensures the fulfillment of our strategic plan to continue expansion and growth in the scope of our activities.”
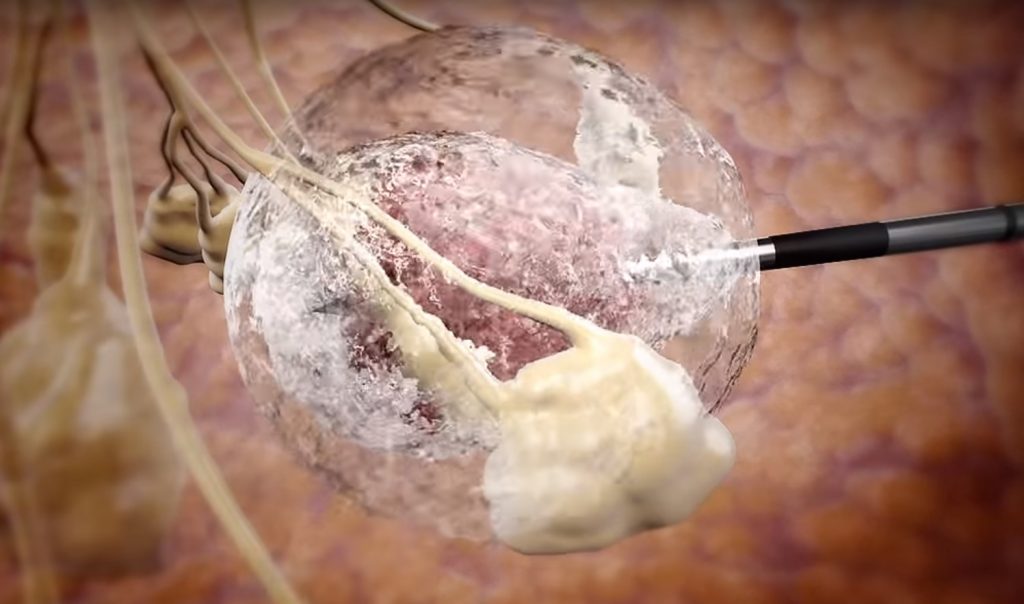
Founded in 2006, IceCure developed a pair of cryoablation systems for treating malignant and benign tumors. The treatments involve streaming liquid nitrogen in a closed circuit and then freezing the tumor with a unique needle developed by IceCure. The company says the healthy tissue remains untouched.
In 2018, IceCure reported great success rates following clinical trials across the US using the IceSense3 system. IceCure said doctors performed the procedures on 146 patients affected by early-stage breast cancer, a majority (103) of whom were under monitoring for almost two years. The company reported that out of the 146 women, one saw the cancer recur.
IceCure touts its procedures as non-invasive, safe, and a viable alternative to surgery. The procedure can be performed in a clinic in about an hour, without the need for general anesthesia or hospitalization.
The company says its progress is driven by the increasing global accelerating trend for minimally invasive treatments, that remove the need for expensive operating rooms and medical staff during surgical procedures. The solution can also help institutions free-up overloaded operating rooms around the world, as well as further prevent and reduce possible infections in medical procedures. These issues are of critical importance during the COVID-19 pandemic.
IceCure’s proprietary, flagship ProSenseTM liquid-nitrogen based system, has FDA approval and a CE mark, according to Shamir.
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments