The European Investment Bank (EIB) has signed a collaboration agreement with the Israel Innovation Authority to pursue investments in companies and initiatives focused on public health. As part of the agreement, the EIB will support Israeli biomed company Pluristem Therapeutics, through its German subsidiary Pluristem GmbH, with a venture debt loan of €50 million ($54 million).
Pluristem Therapeutics develops placenta-derived cell therapy to treat various medical conditions such as infections, inflammation, ischemia (inadequate blood supply to organs), and muscle injury. The company recently said its PLX cell therapy may potentially be used to treat COVID-19 patients with pneumonia and pneumonitis under the compassionate use program, a treatment option that allows for the use of unauthorized medicine for severely ill patients.
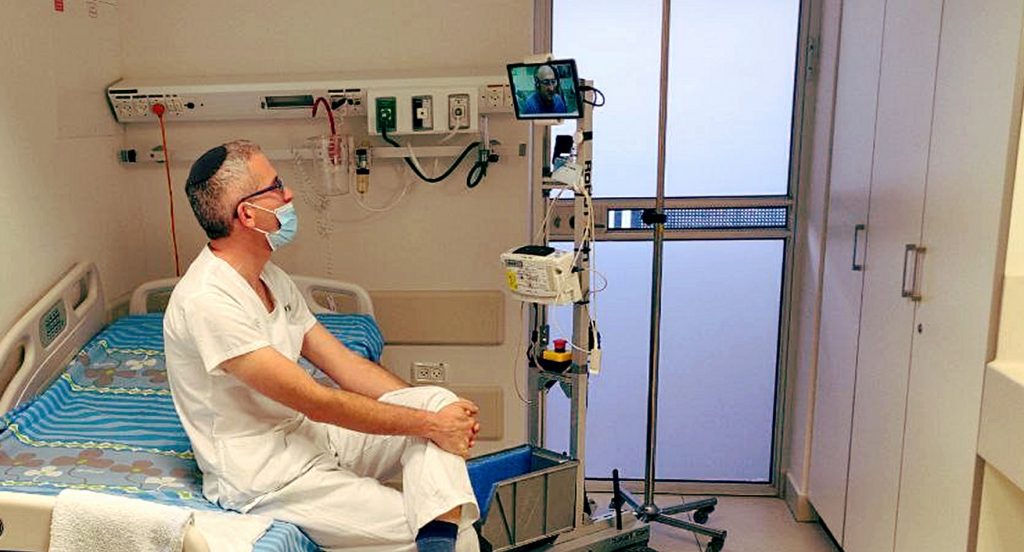
Earlier this month, the first patient suffering from COVID-19 complications was treated with the therapy in the US under the FDA’s Single Patient Expanded Access Program (the compassionate use program). The patient was treated with PLX cell therapy at Holy Name Medical Center in New Jersey, an acute care facility that is currently an active site for Pluristem’s Phase III critical limb ischemia (CLI) study, the company has indicated. Prior to treatment, the patient was critically ill with respiratory failure due to acute respiratory distress syndrome (ARDS) and was under mechanical ventilation in an intensive care unit for three weeks, according to the company.

Pluristem previously treated seven COVID-19 patients in Israel with severe complications under the compassionate use program, with preliminary results showing a 100 percent survival rate, according to the company. In addition, four of the seven patients (66 percent) showed respiratory improvement, and three of the seven were able to start being weaned off ventilators.
Following these studies, Pluristem CEO and President Yaky Yanay said the company was “receiving many inquiries and requests for treatment from healthcare providers and families worldwide.
“In parallel with our planned clinical trial, we expect to continue treating patients under compassionate use through the appropriate regulatory clearances in the United States and Israel, as well as expanding treatment under compassionate use in other countries. Our main focus remains however, the initiation of a multinational clinical study,” he said in early April.
With the EIB financing, Pluristem will pursue research and development in the EU, notably for its regenerative cell therapy platform, and will more forward with its clinical work, the parties said in a joint statement.
Pluristem is set to receive the financing in three parts, subject to pre-agreed clinical regulatory achievements with the first part consisting of €20 million ($21.75 million). The company was previously funded by the Israel Innovation Authority.

The deal is the first Israeli-European project guaranteed by the European Fund for Strategic Investments, the financial pillar of the Investment Plan for Europe, a joint initiative of the EIB and the European Commission. The agreement “aims at deepening the links between Israel and the EU, fostering innovation in the region, closing investment gaps and jointly assuming global leadership in the area of bio-convergence,” the EIB said.
Sign up for our free weekly newsletter
SubscribeA signing ceremony for the deal was held online on Thursday.
“Today’s signatures show that we are successful when we stand together,” said Ambroise Fayolle, EIB Vice-President in charge of innovation.
“Israel is home to a thriving scene of innovative startups, which dominate the high-tech industry. Pluristem is an excellent example of Israeli-European cooperation and EIB support for them is particularly timely, as it will allow the company to develop a treatment for the most vulnerable COVID-19 patients,” he added.

Yanay said the company’s mission “of harnessing the power of regenerative medicine to improve the wellbeing of an aging population has become more urgent than ever, as we work tirelessly to develop a treatment for COVID-19 complications while advancing our portfolio of products in advanced stage trials to treat a number of indications that may positively impact on global healthcare systems.”
Dr. Ami Appelbaum, chairman of Israel Innovation Authority and Chief Scientist at the Ministry of Economy and Industry, said that Israel views bio-convergence as its next economic growth engine.
“This innovative approach serves as an engine to find efficient, diverse, and ingenious approaches to health problem-solving. It integrates biology with engineering, AI, physics, computation, nanotechnology, and material science in order to address unmet needs in numerous industries, including health, agriculture, energy, and defense,” he said.
The collaboration between the European Investment Bank and the Israel Innovation Authority provides a unique opportunity to advance this area providing companies with various financing opportunities from early-stage to growth,” Dr. Applebaum went on.
Related posts

Editors’ & Readers’ Choice: 10 Favorite NoCamels Articles

Forward Facing: What Does The Future Hold For Israeli High-Tech?

Impact Innovation: Israeli Startups That Could Shape Our Future





Facebook comments