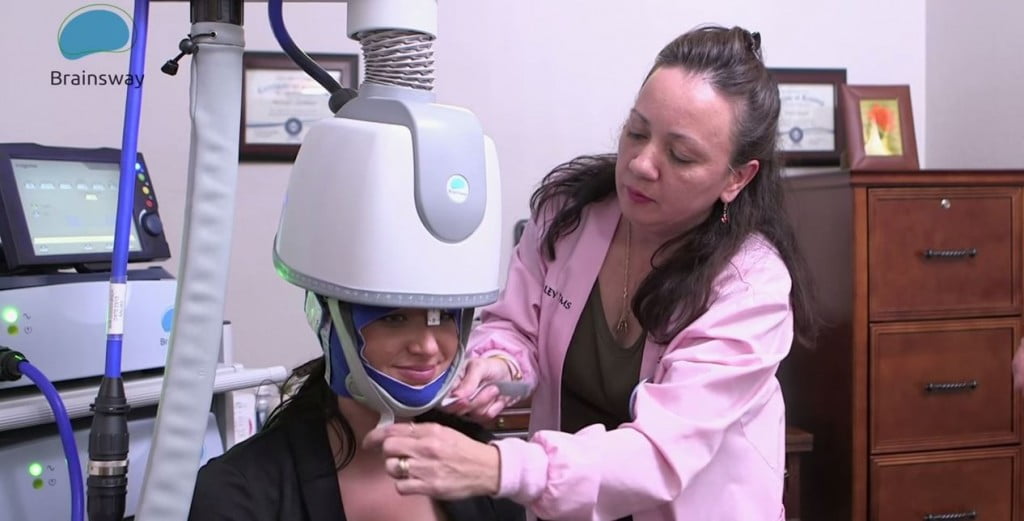
Israeli brain tech startup BrainsWay, which uses deep transcranial magnetic technologies (deep TMS) in the treatment of brain disorders, announced Sunday it has received approval from the US Food and Drug Administration (FDA) for its non-invasive device in the treatment of obsessive-compulsive disorder (OCD).
The approval represents the first-ever approval of a non-invasive medical device for the treatment of OCD, the company said, and the second time BrainsWay’s system was recognized by the federal agency. In May, Brainsway announced it had received 501(k) clearance for its new stimulator to be integrated into BrainsWay’s Deep Transcranial Magnetic Stimulation (Deep TMS) System for the treatment of Major Depressive Disorder (MDD).
“BrainsWay’s OCD certification marks a historic milestone in the treatment of this disorder. This is a new treatment option for patients seeking a meaningful and life-changing OCD solution,” said Dr. Joseph Zohar, a professor of psychiatry at the Sackler School of Medicine at Tel Aviv University and chairman of the International Organization for Compulsive Interference Disorder (ICOCS) and lead author of the OCD trial.
The company plans to begin selling and installing ODS Brainsway helmets immediately. These helmets simulate gray matter in the brain’s prefrontal cortex and engage figure-eight coils to tackle various diseases, including OCD,
schizophrenia, bipolar disorder, and autism.
Brainsway was founded in 2003 by Abraham Zangen, Yiftach Roth, Avner Hagai and David Zacut and has been listed on the Tel Aviv stock exchange since 2007.
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments