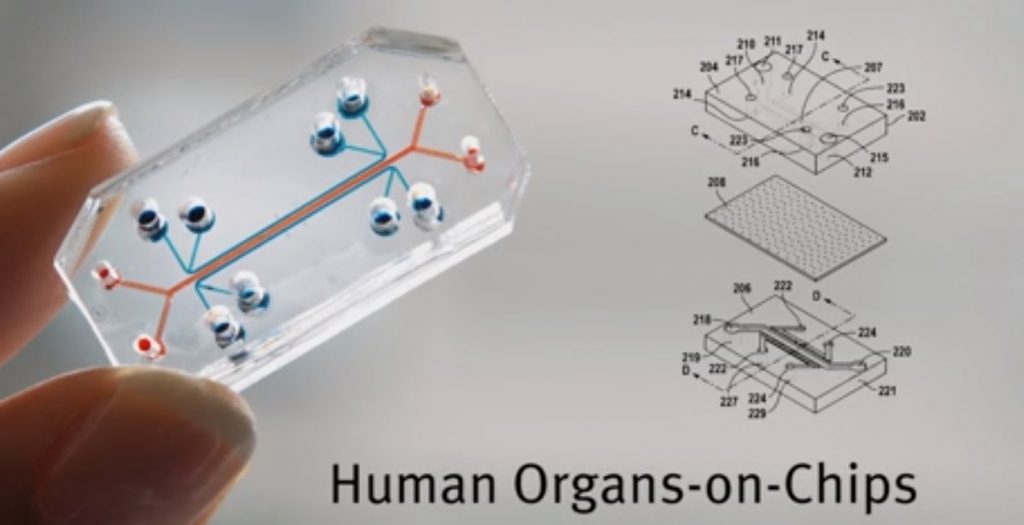
A biotech breakthrough? A team of over 50 researchers at Harvard University and Tel Aviv University (TAU) have successfully built human “organs-on-chips” that they say will allow scientists to better predict human responses to drugs during trials as a way to speed up drug development, and may offer alternatives to some animal testing.
A total of eight microchips were created to recapitulate the build and functions of living human organs – including the lungs, liver, intestines, kidneys, skin, bone marrow, brain, and the blood-brain barrier. The scientists also built an automated instrument to fluidically link up to 10 “organ chips” to create what they called “a functional human Body-on-Chips platform.
The chips and the instrument, called the “Interrogator,” were the subject of two new studies published last week in the science journal Nature Biomedical Engineering. The research was conducted by scientists from Harvard’s Wyss Institute for Biologically Inspired Engineering whose founding director Professor Donald Ingber first developed the “organ-on-a-chip” concept a decade ago, and Tel Aviv University’s Department of Biomedical Engineering and Sagol School of Neuroscience led by Dr. Ben Maoz, a former Technology Development Fellow at the Wyss Institute.
In the first study, titled “Robotic fluidic coupling and interrogation of multiple vascularized organ chips,” the scientists presented the modular Body-on-Chips platform and the Interrogator which can culture the microchips and transfer fluids to mimic normal human blood flow between the different organs of our body. The interrogator enabled the team to culture, perfuse, and link the living human cultured tissues in a multi-Organ Chip system, as well as add and sample the medium in a fully programmable way using the device’s robotic liquid transfer capabilities, while continuously monitoring tissue integrity, according to an explanation of the process provided in a release by the Wyss Institute.
The second study, “Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips,” saw the scientists put the Interrogator to use and apply a new computational model they developed to two different sets of three organ chips – Gut, Liver and Kidney, and Liver, Kidney, and Bone Marrow – linked to each other to test two drugs: nicotine and cisplatin, a common chemotherapy medication.
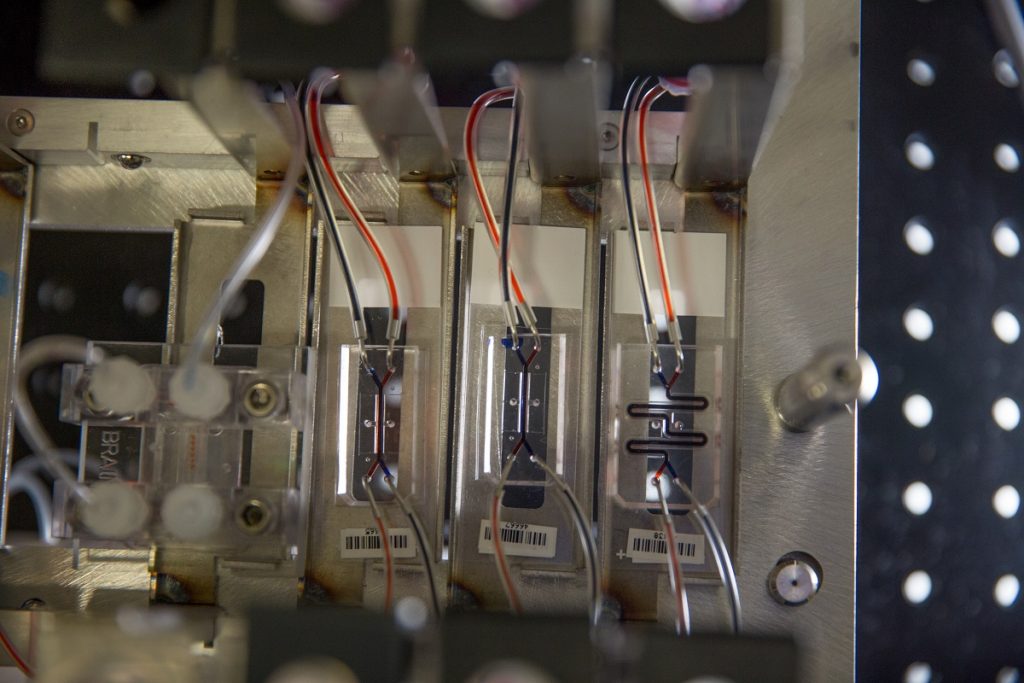
The organs were also linked to a central arterio-venous (AV) fluid mixing reservoir that “helped recapitulate life-like blood and drug exchange between the individual organs, while also providing a way to carry out blood sampling that would mimic blood drawing from a peripheral vein.”
In this study, the researchers accurately modeled the oral uptake of nicotine, which is being investigated as an oral drug for neurodegenerative and inflammatory bowel diseases, and the intravenous uptake of cisplatin, and their first passage through relevant organs with highly quantitative predictions of human pharmacokinetic (involving the quantification of its absorption, distribution, metabolism, and excretion) and pharmacodynamic (involving effects the drug produces on its target organs) parameters.

“The resulting calculated maximum nicotine concentrations, the time needed for nicotine to reach the different tissue compartments, and the clearance rates in the Liver Chips in our in vitro-based in silico model mirrored closely what had been measured in patients,” said Dr. Maoz in a TAU statement.
As for the cisplatin portion of the study, “the analysis recapitulates the pharmacodynamic effects of cisplatin in patients, including a decrease in numbers of different blood cell types and an increase in markers of kidney injury,” said co-first author Anna Herland, Ph.D., who worked on Ingber’s team. “In addition, the in vitro-to-in vivo translation capabilities of the system produced quantitative information on how cisplatin is metabolized and cleared by the liver and kidney, which will make it suitable for more refined predictions of drug absorption, distribution, metabolism, excretion and toxicity.”
Study implications
Both studies highlighted three key recognitions: animal models do not effectively predict drug responses in humans given the fundamental interspecies differences; this leads to high failure rates in clinical trials that test new drugs for their safety and efficacy in humans; drug development is an already highly expensive, arduous, and lengthy process with just 13.8 percent of all tested drugs demonstrating ultimate clinical success and obtaining approval by the Food and Drug Administration (FDA), according to estimated cited by both Harvard and TAU.
“To solve this massive preclinical bottleneck problem, we need to become much more effective at setting the stage for drugs that are truly promising and rule out others that for various reasons are likely to fail in people,” Professor Ingber said in a university statement.
Sign up for our free weekly newsletter
Subscribe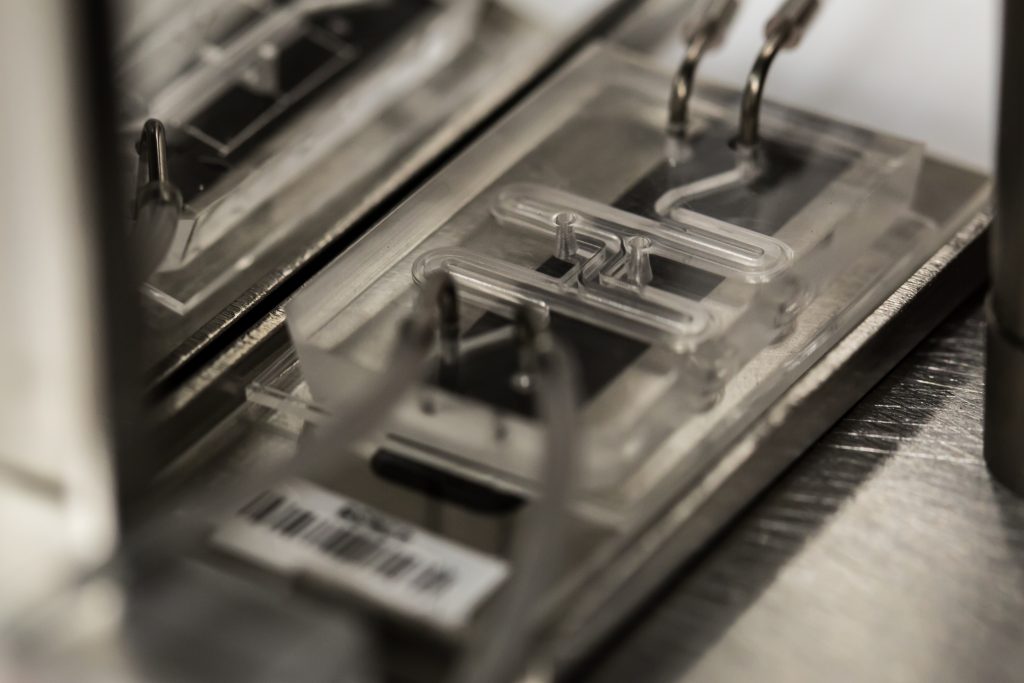
“The modularity of our approach and availability of multiple validated Organ Chips for a variety of tissues for other human Body-on-Chip approaches now allows us to develop strategies to make realistic predictions about the pharmacology of drugs much more broadly,” he added. “Its future use could greatly increase the success rates of Phase I clinical trials.”
Dr. Maoz said the scientists hope their research will help “bridge the gap on current limitations in drug development by providing a practical, reliable, relevant system for testing drugs for human use.”
There is also the question of animal welfare and what the Harvard announcement called the “increasing ethical concerns relating to the use of animal studies.”
Ingber said the team hopes that their “demonstration that this level of biomimicry is possible using Organ Chip technology will garner even greater interest from the pharmaceutical industry so that animal testing can be progressively reduced over time.”
The Organ Chip technology is licensed by a Wyss Institute-launched startup company, Emulate, which is now further developing and commercializing the technology and automated instruments “to bring these important research tools to biotechnology, pharmaceutical, cosmetics, and chemical companies as well as academic institutions and hospitals for personalized medicine,” the institute said.
‘Organs-On-Chips’
Ingber and his team at the Wyss Institute first developed the human “Organ-on-a-Chip” model for the lung, in a study published in Science magazine in 2010. The chips for the additional organs were developed over much of the last decade with teams of collaborators, including the Israeli scientists.
The Organ Chips themselves are made of a clear flexible polymer and contains two parallel hollow channels separated by a porous membrane that allows them to communicate. One channel is lined with cells from a specific human organ or organ structure, the other one is lined with cells presenting a blood vessel.
“Mechanical forces can be applied to mimic the physical microenvironment of living organs, including breathing motions in lung and peristalsis-like deformations in the intestine,” according to an explainer post.
The Organ Chips “are essentially living, three-dimensional cross-sections of major functional units of whole living organs” and “present an ideal microenvironment to study molecular- and cellular-scale activities that underlie human organ function and mimic human-specific disease states, as well as identify new therapeutic targets in vitro.”
“We took a game-changing advance in microengineering made in our academic lab, and in just a handful of years, turned it into a technology that is now poised to have a major impact on society,” Ingber has said.
Related posts

Editors’ & Readers’ Choice: 10 Favorite NoCamels Articles

Forward Facing: What Does The Future Hold For Israeli High-Tech?

Impact Innovation: Israeli Startups That Could Shape Our Future



Facebook comments