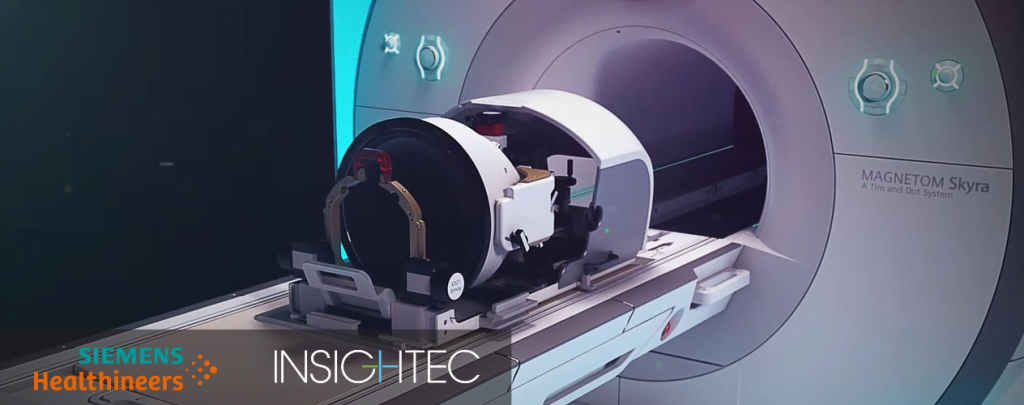
Insightec, a Tirat Carmel-based medical technology company, announced last week that the US Food and Drug Administration (FDA) has approved the company’s Exablate Neuro compatibility for MRI Scanners Magnetom Skyra, Prisma and Prismafit from Siemens Healthineers to treat patients with essential tremor (ET).
Exablate Neuro uses focused ultrasound to precisely target and accurately ablate tissue deep within the brain with no incisions.
Earlier this year, Insightec was among 15 Israeli companies on Fast Company’s “World’s Most Innovative” for revolutionizing surgeries for treating essential tremor, a nerve disorder characterized by uncontrollable shaking, with a non-invasive MRI-guided ultrasound technology. It raised $150 million in a Series E funding round in January.
“This important milestone is directly attributed to the commitment and collaboration of the teams at INSIGHTEC and Siemens Healthineers, who met the challenge head-on,” said Maurice R. Ferré, MD, Insightec CEO and Chairman of the Board, in a company statement. “Expansion of MRI compatibility for Exablate Neuro substantially increases the potential reach of incisionless brain surgery for essential tremor patients.”
Insightech says that more than 1,500 medication-refractory essential tremor patients have been treated around the globe with the tech.
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments