IceCure Medical, a Caesarea-based biomedical company that developed technology which turns cancerous tumors into ice balls, reported this week that clinical trials across the US were showing initial success of up to 99 percent.
Founded in 2006, IceCure has advanced the concept of cryoablation, a process which uses extreme cold to freeze and destroy diseased tissue used by medical experts for years, to develop technology that could be applied to cancer tumors. In 2012, using the company’s IceSense3 system, a minimally invasive cryoablation treatment for breast disease in a non-surgical manner, doctors treated benign breast cancer tumors in four patients during a clinical trial conducted in Kamogowa, Japan. The system had specifically been developed to treat fibroadenomas, which are the most common type of benign breast tumors, typically seen in young women aged 15 to 30.
Now, IceCure is looking at initial results from what it called its “biggest clinical trial” thus far, involving 18 hospitals and medical centers across the US including Columbia University Medical Center and Mount Sinai Beth Israel, both in New York.
Using the IceSense3 system, IceCure said doctors performed the procedures on 146 patients, a majority (103) of whom were under monitoring for almost two years. The company reported that out of the 146 women, one saw the cancer recur.
SEE ALSO: Israeli Startup Freezing Cancer In Its Tracks Now Tackling Kidney, Liver, Bone Tumors
The patients in this recent trial were all older women, with an average age of 76, affected by early-stage breast cancer. The tumors treated were no bigger than 1.5 centimeters in circumference, IceCure said in a statement.
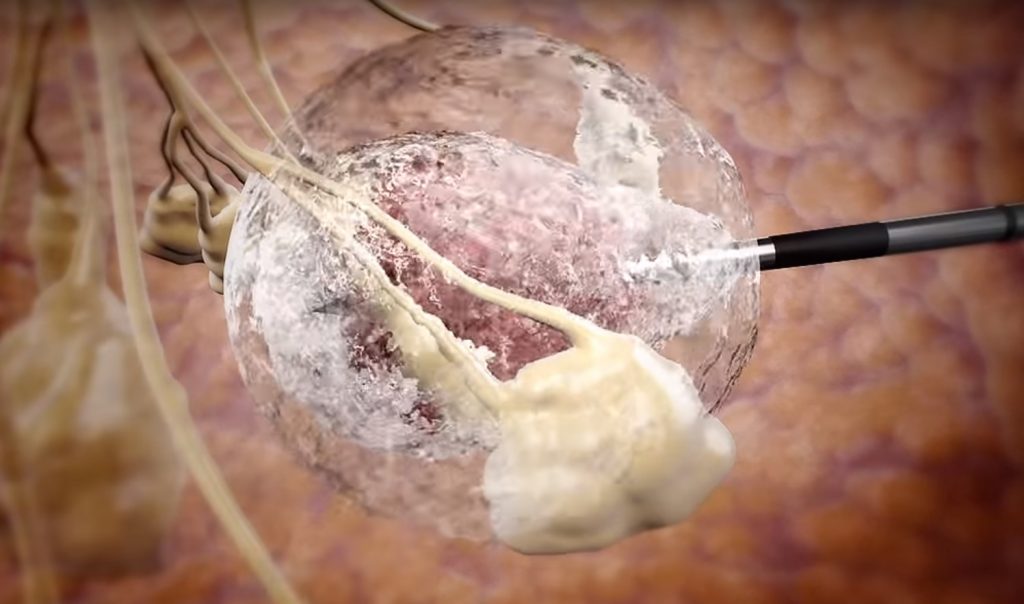
How it works
After administering local anesthesia, a doctor pumps liquid nitrogen at -274F (-170C) through a needle guided by ultrasound scanning to freeze the tumor without affecting surrounding tissue. The whole procedure takes about 15 minutes, can be done in a doctor’s office, and is minimally invasive enough that it does not affect a patient’s daily life.
Indeed, IceCure said that of the 146 patients, 76 percent resumed their daily lives within 48 hours of the procedure.
IceCure CEO Eyal Shamir said that with just one quick, easy treatment, “patients who participated in the trial were able to go back to their lives…without scarring and with no change to the shape and size of the breast.”
The IceSense3 system, he said, “allows for an easy, accurate and safe alternative to the surgical treatment of breast cancer tumors, and allows for the destruction of the whole tumor with cryoablation.”
The initial results, he added, show that the treatment in early-stage breast cancer is an effective, easier and less invasive option that surgery, “without the complications and discomfort of surgery and without hospitalization.”
Shamir said that there was an ongoing, independent trial in Japan since 2014 involving some 300 patients also showed promising initial results.
Sign up for our free weekly newsletter
SubscribeIceCure’s presense and activities worldwide
In March, Shamir told NoCamels that the company has been working to apply the IceSense3 system to treat lung, kidney, liver, and bone tumors, as well. In 2013, the company announced successful clinical trials on patients with malignant lung tumors in Japan.
IceCure provides the answer, Shamir said, as a minimally invasive solution that does not leave permanent damage. Neither does it necessitate the physical removal of a tumor from the body, as the body absorbs the dead cells over time.
“IceCure did not invent the cryoablation method, which has been known for over twenty years, but it is the most advanced product [of its kind] on the market,” Shamir said at the time. While other freezing technologies can reach temperatures as low as IceCure, they do so much more slowly and many cannot keep a stable freezing temperature. He explained that IceCure not only reaches freezing temperatures four times faster, but can also keep a colder stable temperature for at least forty times longer.
IceSense3 has FDA and CE approval as well as approval from various other local standards, including Hong Kong, Singapore, Thailand, and Mexico.
IceCure’s end users include mostly surgeons, and the company has an office in Collierville, Tennessee that works directly with doctors in the US. The company works through local distributors in other countries.
IceCure’s History
Before 2006, IceCure was part of an incubator hosted by the Israel Innovation Authority (then the Israel Chief Scientist Office) to find a minimally invasive alternative to treat cancer. It became a private company in 2008 before going public in 2011. During most of its years in operation, IceCure focused on research and development and its sales aspect is still in its early stages. Shamir became CEO in 2016, following the departure of previous CEO Hezi Himelfarb, who is now general manager and COO at robotic medical technology company Microbot Medical.
SEE ALSO: New Device Destroys Breast Tumors With Extreme Cold
As of 2011, IceCure is a publicly traded company on the Tel Aviv stock exchange, with a market cap of almost NIS 31.5 million ($9.5 million). A majority of its shares (70 percent) are owned by Chinese investor Haixiang Lee, founder and managing partner at VI Ventures.
Ido Levy contributed to this report.
Related posts

Editors’ & Readers’ Choice: 10 Favorite NoCamels Articles

Forward Facing: What Does The Future Hold For Israeli High-Tech?

Impact Innovation: Israeli Startups That Could Shape Our Future




Facebook comments