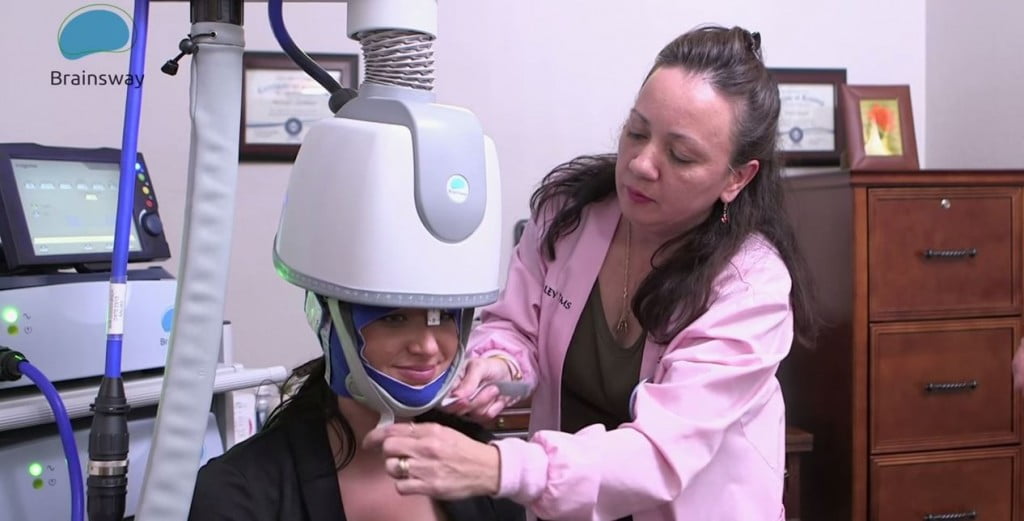
 May 9, 2018 | Israeli company Brainsway, a leader in the advanced non-invasive treatment of brain disorders, announced this week that it received 510(k) clearance from the US Food and Drug Administration (FDA) for its new stimulator to be integrated into Brainsway’s Deep Transcranial Magnetic Stimulation (Deep TMS) System for the treatment of Major Depressive Disorder (MDD). Deep TMS simulates relevant areas of the brain with non-invasive treatment using electromagnetic waves running through a special H-Coil helmet. This reaches deeper and larger surface areas of the brain than standard TMS treatments. The simulator enhances the entire Deep TMS system, streamlining treatment for physicians and their patients. It also provides enhanced features focusing on the ease of use for the physician. Features include a patient management system with built-in depression treatment protocol and a user-friendly tool for accurate, rapid motor threshold detection. “Brainsway’s new stimulator represents a very important product advancement that solidifies the company’s leading innovative position within the US market. Furthermore, it sets the foundation for Brainsway to become a fully integrated company that develops, produces and commercializes our innovative, non-invasive medical systems for the treatment of brain disorders globally,” said CEO Yaacov Michlin, in the statement.
May 9, 2018 | Israeli company Brainsway, a leader in the advanced non-invasive treatment of brain disorders, announced this week that it received 510(k) clearance from the US Food and Drug Administration (FDA) for its new stimulator to be integrated into Brainsway’s Deep Transcranial Magnetic Stimulation (Deep TMS) System for the treatment of Major Depressive Disorder (MDD). Deep TMS simulates relevant areas of the brain with non-invasive treatment using electromagnetic waves running through a special H-Coil helmet. This reaches deeper and larger surface areas of the brain than standard TMS treatments. The simulator enhances the entire Deep TMS system, streamlining treatment for physicians and their patients. It also provides enhanced features focusing on the ease of use for the physician. Features include a patient management system with built-in depression treatment protocol and a user-friendly tool for accurate, rapid motor threshold detection. “Brainsway’s new stimulator represents a very important product advancement that solidifies the company’s leading innovative position within the US market. Furthermore, it sets the foundation for Brainsway to become a fully integrated company that develops, produces and commercializes our innovative, non-invasive medical systems for the treatment of brain disorders globally,” said CEO Yaacov Michlin, in the statement.
Related posts

Top Tel Aviv Hospital Using AI In Neurological Therapy





Facebook comments