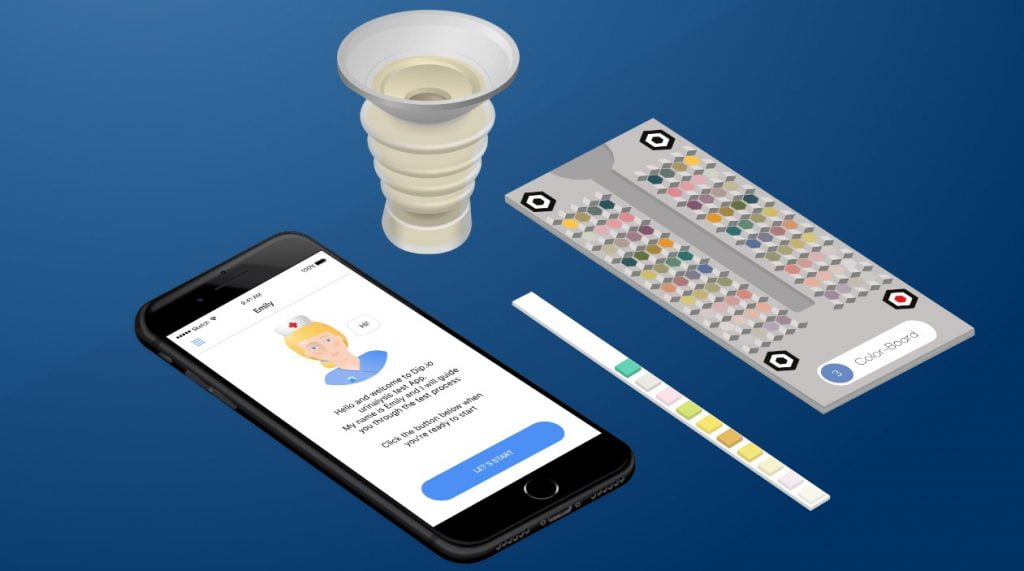
![]() April 15, 2018 | Israeli startup Healthy.io announced a new partnership with the US National Kidney Foundation (NKF) and Geisinger, one of the largest health services organizations in America, for a clinical trial using a smartphone-enabled home urinalysis device for chronic kidney disease (CKD) among patients with high blood pressure. Healthy.io, according to the Tel Aviv-based company, “uses computer vision, machine learning, and user-centric design to turn the smartphone camera into a medical device.” The Israeli startup’s app will enable users to conduct a urinalysis test at home and securely share results with their clinicians to monitor the presence of albumin, which indicates kidney damage. Part of the clinical trial will be to “examine the effect of mailed, Healthy.io smartphone urinalysis kits (Dip.io test) to improve albuminuria screening compliance and detection of albuminuria.,” the companies said in a statement. Healthy.io CEO Yonatan Adiri said the company was “proud to pioneer its ‘adherence as a service’ platform with such forward-looking institutions as Geisinger and the National Kidney Foundation, adding that its “mission is to use advanced computer vision and patient-centric design to let clinicians empower their patients at scale without additional cost or effort.” He went on, “Like a Netflix for adherence we lean on the spread of digital technology and efficient logistics to offer on-demand testing delivered directly to the home. With a smartphone in your pocket, the point of care becomes wherever you are.” Alexander Chang, M.D., practicing nephrologist and assistant professor in the Kidney Health Research Institute at Geisinger, said, “Early detection of CKD is crucial so that risk factors can be aggressively managed to prevent end-stage renal disease and cardiovascular disease.” Kerry Willis, Ph.D., Chief Scientific Officer at NKF said the new trial “will provide important information on how to increase testing for CKD in this high-risk population.Our hope is that a home-based test makes it easier for patients at risk for CKD to comply with regular albuminuria screening and that this will lead to earlier diagnosis and treatment of CKD, reducing cardiovascular risk and preserving kidney function.” The clinical trial will get underway on April 16.
April 15, 2018 | Israeli startup Healthy.io announced a new partnership with the US National Kidney Foundation (NKF) and Geisinger, one of the largest health services organizations in America, for a clinical trial using a smartphone-enabled home urinalysis device for chronic kidney disease (CKD) among patients with high blood pressure. Healthy.io, according to the Tel Aviv-based company, “uses computer vision, machine learning, and user-centric design to turn the smartphone camera into a medical device.” The Israeli startup’s app will enable users to conduct a urinalysis test at home and securely share results with their clinicians to monitor the presence of albumin, which indicates kidney damage. Part of the clinical trial will be to “examine the effect of mailed, Healthy.io smartphone urinalysis kits (Dip.io test) to improve albuminuria screening compliance and detection of albuminuria.,” the companies said in a statement. Healthy.io CEO Yonatan Adiri said the company was “proud to pioneer its ‘adherence as a service’ platform with such forward-looking institutions as Geisinger and the National Kidney Foundation, adding that its “mission is to use advanced computer vision and patient-centric design to let clinicians empower their patients at scale without additional cost or effort.” He went on, “Like a Netflix for adherence we lean on the spread of digital technology and efficient logistics to offer on-demand testing delivered directly to the home. With a smartphone in your pocket, the point of care becomes wherever you are.” Alexander Chang, M.D., practicing nephrologist and assistant professor in the Kidney Health Research Institute at Geisinger, said, “Early detection of CKD is crucial so that risk factors can be aggressively managed to prevent end-stage renal disease and cardiovascular disease.” Kerry Willis, Ph.D., Chief Scientific Officer at NKF said the new trial “will provide important information on how to increase testing for CKD in this high-risk population.Our hope is that a home-based test makes it easier for patients at risk for CKD to comply with regular albuminuria screening and that this will lead to earlier diagnosis and treatment of CKD, reducing cardiovascular risk and preserving kidney function.” The clinical trial will get underway on April 16.
Related posts

Israeli AI Safety Tool Among TIME’S Best Inventions For 2024

TAU Team Discovers Mechanism To Eliminate Cancerous Tumors

Ashdod Port Investing In Startups As Part Of Innovation Strategy



Facebook comments