A model embryo grown in the lab and not the womb, using stem cells and not egg and sperm, could be the future of research into pregnancy and drug testing and even provide bespoke organ transplants, its creator says.
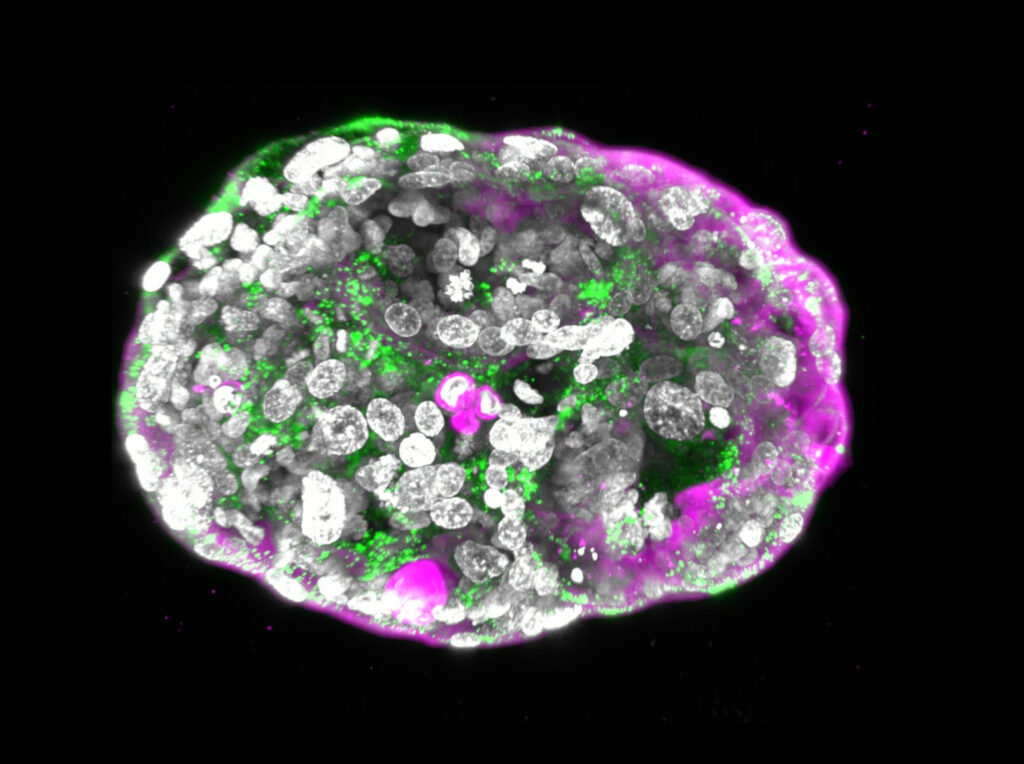
What Prof. Jacob (Yaqub) Hanna of the Weizmann Institute of Science and his team have created is not a viable baby, he tells NoCamels, but rather a model of an embryo mimicking the stage of development a fetus in utero would have reached by day 14.
All of the actual development of a fetus happens in the first weeks, Hanna explains. After that, a pregnancy is “just growth,” he says.
Because these first weeks are so integral to embryonic development, this is the period in which the majority of nonviable pregnancies fail.
“Most of the developmental defects happen by week five,” Hanna says. “We detect them later, but they happen very early.”
He explains that studying the early weeks of pregnancy is crucial to understanding these developmental defects, but most women do not even know they are pregnant at this stage.
And even when they do know, Hanna says, “there is no ethical justification” for removing tissue from the embryo for research that would in any event require hundreds of thousands of samples.
“One sample here, one sample there – although we are desperate, this is never going to be enough,” he says.
It was the need to understand these crucial first few weeks of gestation that first led Hanna to build the model embryo using stem cells. The models can then be used to try to understand why defects develop and also to observe the impact of new medications and therapies on an embryo.

The models are constructed in two ways: either by using tissue from embryos that were donated decades ago or by taking a skin or a blood cell and “erasing everything in it to go back to embryonic stem cell state.”
The latter method was developed by Prof. Shinya Yamanaka of Kyoto University, and earned him a 2012 Nobel Prize.
It is also the preferred method to construct the model, Hanna explains, as it contains the genetic DNA of the cell donor, particularly in cases in which the models will be used to develop organ tissue for transplantation.
Hanna says that in human pregnancy, organ development – organogenesis – begins at day 15, and is expected to be completed by week eight.
Tailor-Made Organs
Hanna explains that he and his team at the Rehovot-based Weizmann Institute are now working on a model embryo that replicates development at a later stage than the current 14 days, with the aim of generating organ tissue for transplantation.
He clarifies that the process of growing any organ begins at day 15 and while it takes almost the entire pregnancy to fully complete, by day 40 “all the ingredients” are there.
“Then you just wait for them to mature, proliferate, mature more. But all the cell types, all the layers of all the organs are there,” he says.
Sign up for our free weekly newsletter
SubscribeHanna gives the example of a leukemia patient who is facing death because he cannot find a blood cell donor who is a match.
A skin cell from that patient can be transformed into a naïve stem cell (stem cells that have not matured into a specific form) and from that a model embryo can be developed to 30 or 33 days, complete with bone marrow, where the blood cells are made, for transplantation.
“We have his identical stem cells, can do a bone marrow transplant and save his life,” Hanna says.
He believes this to be the best way to obtain all the different cell types that are needed by different patients.
“Rejection is impossible because it is the same DNA [with] no cells from a donor and we save his life. That is our dream, and we think it’s feasible,” he says.
And so convinced is Hanna that this is the future of medicine, he and several peers have founded a medtech company in this field, called RenewalBio.
Ethical Issues
Hanna is firm that the model embryo absolutely cannot grow into a viable fetus, and is incapable of feeling pain or approaching any form of cognitive development at all.
“We call them developmental restricted cells,” he says of the cells used to develop the model. This involves removing a single gene so that “there is absolutely no neural tissue.”
Do not assume that the model embryos are identical to human embryos, he stresses. “They’re not; there are differences.”
He says that the team even sought counsel from local rabbis, imams and priests on the ethical aspects of the research.
“This cannot be defined as a human being because it can never be born,” he says.
Hanna draws a parallel between his work and IVF treatment, whose introduction in the 1970s was also met with questions and uncertainty. He explains that an embryo can only be implanted into the uterus when it is less than 60 cells, and by day 5, an embryo already comprises 70 to 100 cells.
“It’s more of an aggregate of important tissue,” he says.

According to Hanna, no other researchers are working on the same principles as him and his team, but he is definite that the process, particularly because of its sensitive nature, should be open for everyone to see and evaluate. The research was recently published in the Nature journal.
“We report as soon as we have results; we actually want to hear back from the public,” he says. “We want the public to know that nothing is done in the shadows or the dark.”
Related posts

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Israeli Device Is New, Drug-Free Solution For Men Coping With ED




Facebook comments