For people living with advanced kidney failure, hemodialysis treatment can be a lifeline to a normal existence.
A MedTech company in Israel has created a contactless, robotic device to ensure that the hemodialysis process runs as smoothly as possible – without a very common side effect that can disrupt treatment and threaten the health of the patient.
Hemodialysis uses a machine to clean waste from a person’s blood when the kidneys can no longer do this vital job, but the way in which the machine accesses the blood can cause its own set of medical issues.
The human kidney, which is roughly the size of a computer mouse, filters the blood in the body every half hour. Without that process – or the medical alternative – a person is at elevated risk of high blood pressure, heart disease and even stroke or death.
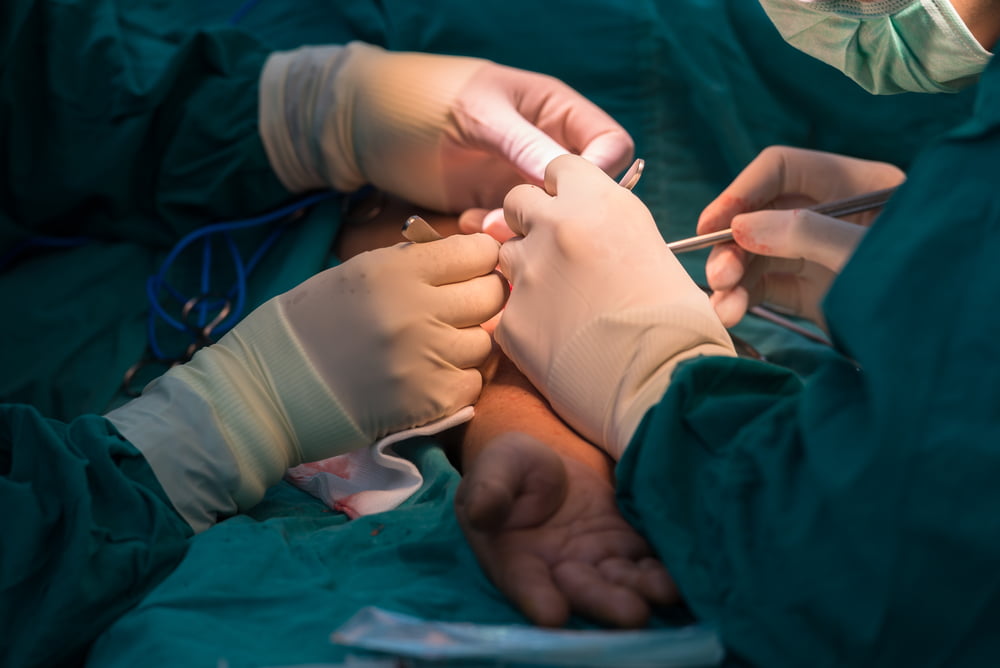
Each hemodialysis treatment takes around four hours, three to five times each week, with access to the blood gained through a surgically created portal, called a fistula, in the patient’s arm.
To create the fistula, the patient must undergo vascular surgery to connect an artery to a vein, explains Shai Policker, veteran medical entrepreneur and board member at PatenSee, the startup behind the innovation.
Invasive surgery aside, Policker tells NoCamels, each fistula takes several months to “mature” and be ready for use in dialysis. Those fistulas, he says, are the lifeline of the dialysis patient.
The fistula is used to pump unfiltered, deoxygenated blood out of the body into the dialysis machine and the cleaned blood back in. But because it is artificially created, the fistula is vulnerable to a narrowing of the blood vessels that has no external signs, known as asymptomatic stenosis.
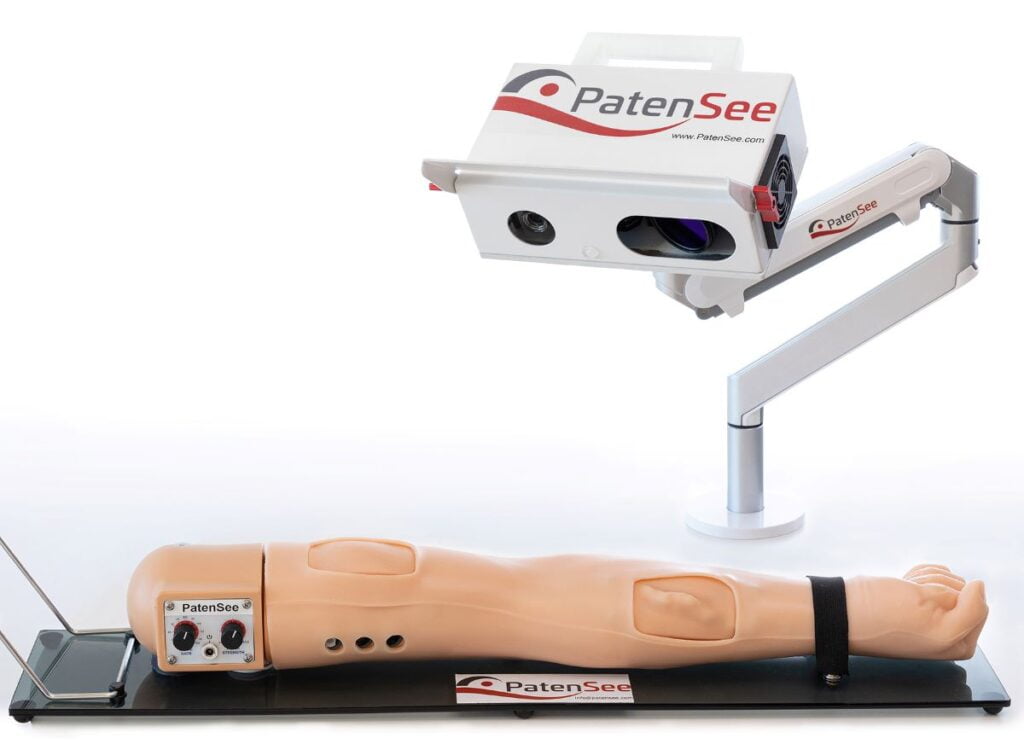
PatenSee has developed a contactless, portable device that uses AI to inspect the ongoing condition of the fistula and alert medical professionals to early signs of stenosis – allowing for preventive measures rather than corrective action once the blood vessels have already started to narrow.
When stenosis occurs, the blood flow through the fistula slows, adversely impacting the pace and quality of the treatment, making the process more painful or even causing a dangerous blood clot.
Without detection, stenosis can also cause the fistula to become completely blocked, rendering it useless and forcing the patient to undergo another round of painful surgery that requires days of hospitalization.
“We are using veins as if they are arteries,” Policker says. “Sometimes it will block the fistula and the blood vessels within two or three months. Sometimes it can work well for a year or a year and a half and only then be lost. But in almost all cases something will happen. Something like 20 to 30 percent of patients will get some vascular access event within a year. So it’s a big issue.”
While there are no immediate visible symptoms, trained medical personnel can manually check for signs of stenosis, a “look, listen and feel” process that monitors the flow of blood through the fistula and, according to Policker, takes just 10 minutes or so.
But this is often not possible due to staffing constraints in a busy renal unit, especially in the United States where Policker says each clinic can treat up to 30 hemodialysis patients at a time.
“It’s like a factory,” he says.
Even when these checks do take place, they involve physical contact with a painful area in an immunocompromised patient, putting them at risk of infection.

No Contact, No Risk
PatenSee’s device, which is mobile and can be used anywhere, requires no physical inspection of the fistula and takes a fraction of time of the manual process.
Sign up for our free weekly newsletter
SubscribePolicker says he became aware of the issue several years ago, and as part of his role as CEO of Israeli MedTech incubator MEDX Xelerator challenged entrepreneurs to present solutions to the problem.
PatenSee came up with the idea of replacing the entire physical exam with a set of optical and sound tests carried out mechanically.
The first part of the test is to create 3D imaging of the fistula, which involves analyzing its contours when the arm is in various positions.
The second part is the use of a laser vibrometer, which Policker likens to an eavesdropping device used in spy movies. This device “listens” to the blood flow through the fistula on several frequencies.
Both parts collate the results using AI and present an image of the state of the fistula that Policker says is at least as accurate as a manual check by a trained clinician. What is more, the machine can be operated by someone with minimal training, and the results sent for inspection by a physician.
Should the examination reveal early stages of stenosis, the patient can be referred for a 20-minute procedure that reopens the fistula.
The device has undergone successful preclinical trials in an Israeli hospital and Policker says the company is now in talks with several large dialysis providers around the world, including in Europe and the United States.
There is high demand from medical institutions around the world to host the clinical trials, he says.
According to the International Society of Nephrology, more than 850 million around the world suffer from some form of kidney disease – twice the global number of diabetes patients. Of that number, up to 10 million need hemodialysis or a transplant.
In the United States alone, around 800,000 people are living with renal failure, of whom more than half a million are receiving dialysis treatment. The American Kidney Fund lists renal disease as one of the top 10 causes of death in the US, leading to more annual fatalities than breast or prostate cancer.

Policker says that the device could be used in the future to check for thrombosis (blood clots) in other parts of the body, but for now the focus is on fistula used in hemodialysis.
There are other medtech companies looking for solutions to stenosis in fistulas, but Policker says that while a number of them are “reasonably good,” they are not in widespread use as they still involve touching the patient – risking infection and causing discomfort.
PatenSee received funding from an incubator that got it through the development stage and initial trials, and is now raising additional funding from private investors to finance the clinical trials.
He believes that the device should be ready to market within two years or so. While it will be initially pricey, he concedes, “there is an ability to take that hardware and make it very small and very cheap.”
Acquiring approval from the US Food and Drug Agency (FDA) and its counterpart the European Medicines Agency (EMA) should be a “relatively simple regulatory process,” Policker explains, as the device requires no physical contact with the patient at all.
PatenSee’s innovation, he says, “gets rid of those emergency life-threatening situations.”
Related posts

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Israeli Device Is New, Drug-Free Solution For Men Coping With ED




Facebook comments