In a world first, Israeli scientists say they have engineered 3D human spinal cord tissues and implanted them in an animal lab model with long-term chronic paralysis.
The scientists said the “highly encouraging” results showed that there was an 80 percent success rate in restoring walking abilities for patients with chronic paralysis.
The research from Tel Aviv University was revealed on Monday after publication in the peer-reviewed scientific journal Advanced Materials.
Scientists engineered spinal cord tissue from human cells and implanted them into mice. One group had mice that had recent paralysis and the other had mice that had had paralysis long-term. For the mice, a six-week paralysis was the equivalent of a human living with paralysis for six months to a year.
Fifteen mice were implanted with long-term paralysis. Twelve of those mice went through a rapid rehabilitation process where they walked normally just three months later, according to the study.

This is said to be the first instance in the world in which implanted engineered human tissues have generated recovery in an animal model for long-term chronic paralysis, which is the most relevant model for paralysis treatments in humans.
Professor Tal Dvir, who led the research team from the Sagol Center for Regenerative Biotechnology at TAU, tells NoCamels his team is looking to start human clinical trials in about two and a half years. “We have discussed the research plan with the FDA [US Food and Drug Administration]. It’s exciting because it can eventually reach clinical trials actually help people, not just work on animals.”
“People who are paralyzed will have hope that they will walk again,” he adds.
Prof. Dvir said the scientists took a small biopsy of belly fat tissue from a patient and separated the cells after which they used genetic engineering to program the cells to revert them to a state that resembles embryonic stem cells, or stem cells capable fo becoming any type of cell in the body.
The team was able to create a personalized hydrogel from the materials and they put the stems cells in the hydrogel to mimic embryonic development of the spinal cord, Prof. Dvir tells NoCamels.
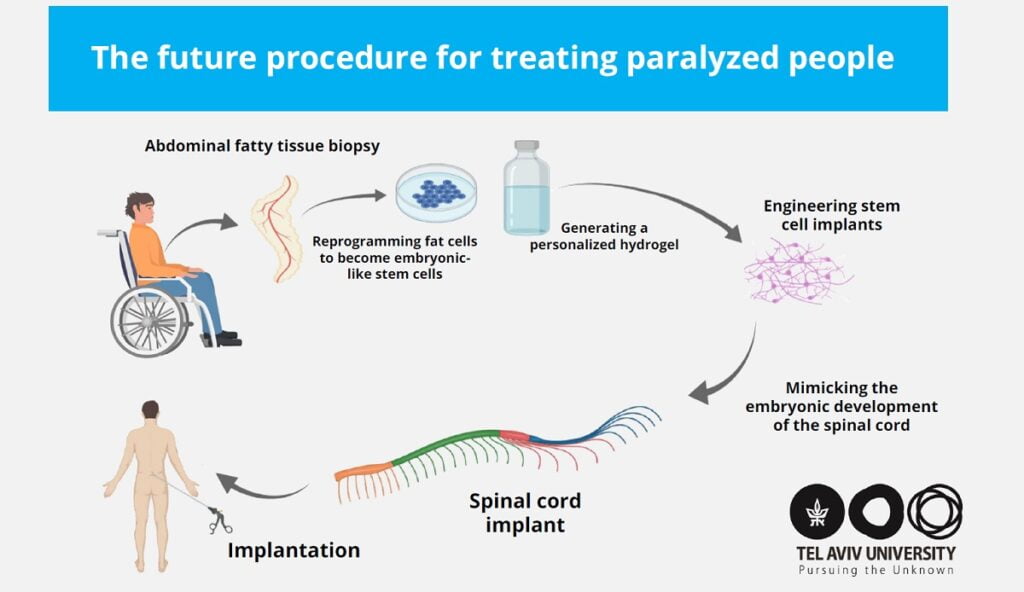
“We removed the infected tissue and transplanted the engineered tissue,” Dvir says, “The small tissue is an implant which basically forms a piece of the spinal cord.”
Mimicking the embryonic development of the spinal cord turned the cells into 3D implants of neuronal networks containing motor neurons, Prof. Dvir said.
Sign up for our free weekly newsletter
SubscribeProf. Dvir’s team includes PhD student Lior Wertheim, Dr. Reuven Edri, and Dr. Yona Goldshmit. Other contributors included Prof. Irit Gat-Viks from the Shmunis School of Biomedicine and Cancer Research, Prof. Yaniv Assaf from the Sagol School of Neuroscience, and Dr. Angela Ruban from the Steyer School of Health Professions, all at Tel Aviv University.
Tissue implant tech
In November 2018, Israeli researchers, which included Prof. Dvir, then-postdoctoral researcher Reuven Edri, and then-doctoral students Nadav Noor and Idan Gal of TAU, in collaboration with Professor Dan Peer and Professor Irit Gat Viks of TAU’s Department of Cell Research and Immunology and Professor Lior Heller of Assaf HaRofeh Medical Center said they invented the first fully personalized tissue implant engineered from a patient’s own biomaterials and cells, paving the way for new technology that would make it possible to develop any kind of tissue implant from one small fatty tissue biopsy.
The development would greatly reduce the risk of an immune response to an organ transplant, the scientists said at the time.
In April 2019, the scientists, including Dvir, used the same innovative technology to create a live heart using a revolutionary 3D printing process that combines the human tissue taken from a patient.
“The technology is the same – we’re taking the same fatty tissue, performing the genetic engineering, differentiating the cells. It’s a platform we can apply to any tissue type,” Dvir said, adding the only difference is the kind of cells that were created to make the heart tissue, and that they don’t need to 3D print tissue for the spinal cord.

The process involved taking fatty tissue, after which the cellular and a-cellular materials were then separated. While the cells were reprogrammed to become pluripotent stem cells and efficiently differentiated to cardiac or endothelial cells, the extracellular matrix (ECM), a three-dimensional network of extracellular macromolecules, such as collagen and glycoproteins, were processed into a personalized hydrogel that served as the printing “ink,” Tel Aviv University said in a statement.
The differentiated cells were then mixed with the bio-inks and were used to 3D-print patient-specific, immune-compatible cardiac patches with blood vessels and, subsequently, an entire, tiny heart.
“With our technology, we can engineer any tissue type, and after transplantation, we can efficiently regenerate any diseased or injured organ — a heart after a heart attack, a brain after trauma or with Parkinson’s disease, a spinal cord after injury,” said Dvir at the time. “In addition, we can engineer adipogenic (fatty tissue) implants for reconstructive surgeries or cosmetics. These implants will not be rejected by the body.”
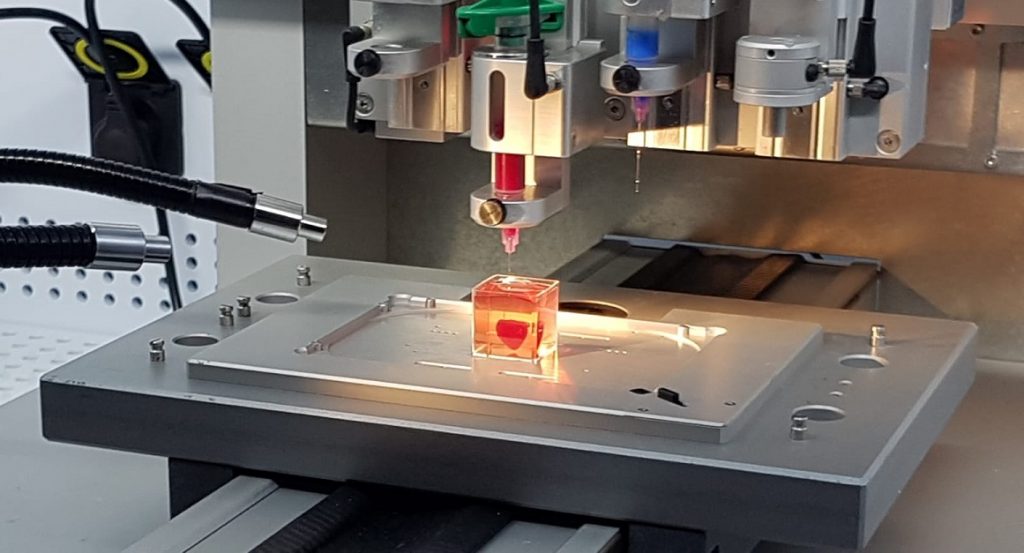
That same year, Dvir and Alon Sinai established Matricelf, a biotech company developing this platform to create cell and tissue implants for a wide range of medical conditions. The company was formed to move the platform towards clinical trials.
“We have many patents in the company already, which were licensed from Tel Aviv University. The company works in parallel with TAU’s tech transfer company Ramot. The tech was transferred from the lab to the company,” Dvir said.
With offices located in Ness Ziona, the company has 10 employees and has raised NIS 24 million (about $7.5 million) to date.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer



Facebook comments