Just weeks after Bonus BioGroup reported an eye-popping survival rate of 94 percent of severely ill COVID-19 patients treated with its cell-based drug product MesenCure in a Phase II clinical trial, interest in the Israeli biotechnology company’s research is spreading.
The company grabbed global headlines late last month with data showing its drug’s effectiveness in treating severe COVID-19 patients at risk of death and that using its treatment could reduce hospitalization stays by about half compared to a control group.
According to its report, of the 50 hospitalized severe COVID-19 patients who participated in the clinical trial and received MesenCure, 47 survived and recovered. That statistic put Bonus BioGroup’s name in the news. And their cell-based drug aimed at curing patients affected by acute respiratory distresses, was even dubbed a “miracle drug.”
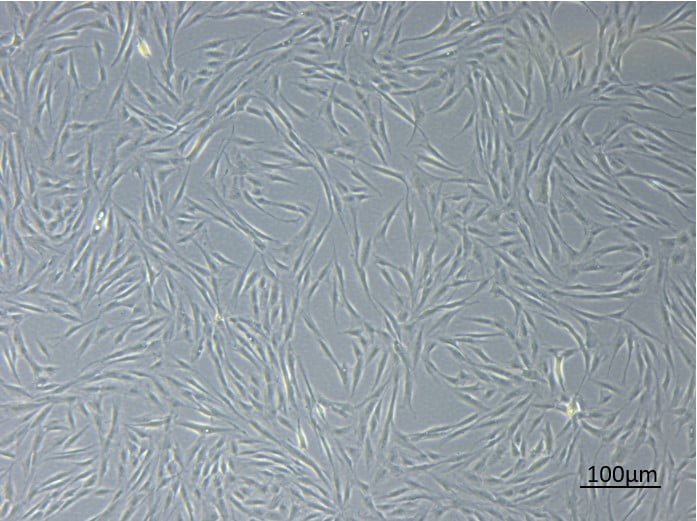
“Today most drugs are not dealing with curing diseases but with managing diseases,” Dr. Tomer Bronshtein, Head of Research at the Haifa-based biotech firm tells NoCamels. “Now, cell therapy…has the ability to really cure a disease. I think MesenCure is a very good demonstration for such a therapy that actually cures the patients.”
A drug that targets inflammation
The effectiveness of MesenCure springs from its main biochemical component, mesenchymal stromal cells. These cells are known for their immuno-modulatory ability, which acts on the inflammation in the lungs following an overreaction of the immune system.
“Patients are not dying because of the virus itself,” says Shai Meretzki, CEO and president of Bonus BioGroup, “What is killing the patient is a process called ‘cytokine storm’ which blocks the lungs of the patient, preventing him from breathing and bringing him into a life-endangering situation.”
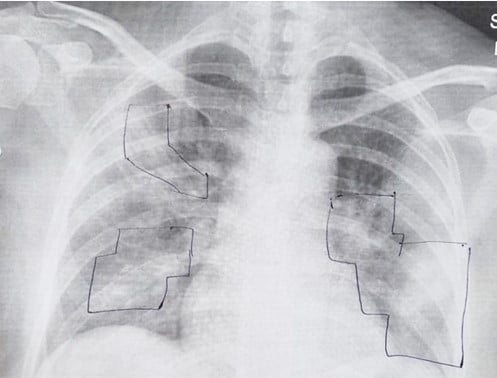
Here’s how it works: when injected into the patient’s body, the mesenchymal cells sense the inflammation, and begin releasing an array of anti-inflammatory proteins that lower the immune cells’ count in the lungs, transferring them instead back into the bloodstream where they need to be to fight the virus itself.
By fighting the inflammation, Dr. Bronshtein says, the variant of COVID-19 or the type of respiratory distress the patient is affected by ceases to matter. MesenCure “will still do the job” regardless of whether the virus is called Delta, Omicron, or is named after “any subsequent letter of the Greek alphabet,” he adds.
SEE ALSO: Israeli Researchers Say Medical Cannabis Could Effectively Treat Some COVID-19 Symptoms
“You are getting a very sophisticated modality to treat Covid-19,” Dr. Bronshtein tells NoCamels. “Instead of one drug that acts on one target … which suppresses the entire immunity and also prevents the body from continuing to fight the virus, think that now you have an intelligent creature inside you that knows how to act, when to act, and where to act.”
There are other studies with other anti-inflammatory drugs underway for the treatment of severe COVID-19 patients, of course. The EU’s latest approved anti-inflammatory drug Actemra, for example, showed it could reduce mortality rates in a test group of 2,022 participants to 31 percent, as opposed to a mortality rate of 35 percent for 2,094 participants in a control group.
Sign up for our free weekly newsletter
SubscribeBonus BioGroup’s clinical trial may have included a small sample of severe COVID-19 patients but the data was impressive, showing a mortality rate that fell from about 23 percent in the control group to less than seven percent in the test group, according to Bronshtein.
The innovation in MesenCure has to do with how mesenchymal cells are “educated.”
Before injection, mesenchymal cells are harvested from the fat tissue of a healthy donor and stored in a so-called cell bank. Bronshtein says the cells are then multiplied and undergo an “education” process which subjects them to the conditions encountered in the inflamed organ part. In doing so, their potency and sophistication in fighting inflammation is improved.
“This ‘education’ is the heart of the innovation of MesenCure,” says Bronshtein.
According to the US National Institutes of Health, while research has been conducted on the regenerative and immunomodulatory properties of mesenchymal cells, its full applicability in the fight against COVID-19 remains to be tested. A recent report cited that studies in the use of mesenchymal cells for COVID-19 treatment remain small and non-standardized due to different country regulations concerning experimentation.

So, if studies are still small, why the excitement over MesenCure?
Bronshtein says that, while it is unlikely that this will be the “magic bullet” that will eradicate all our virus-related problems, it may still provide a significant, long-awaited protection layer for those patients that are liable to suffer from severe respiratory distress syndromes currently not covered by the antiviral drugs market.
And with the Omicron variant now wreaking havoc around the world – and healthcare officials warning of new coronavirus variants on the horizon – a promising drug means there’s room for hope.
Currently, Bonus BioGroup has filed a request to have MesenCure approved for emergency treatment used both in Israel and abroad and plans to conduct further trial phases in the near future.
“We want to advance the option that this will become available to as many people as possible as soon as possible, especially since we can see that MesenCure is so effective on the one hand…, and, no less important, we know that MesenCure is safe,” says Bronshtein.
Related posts

Israeli Medical Technologies That Could Change The World

Harnessing Our Own Bodies For Side Effect-Free Weight Loss

Missing Protein Could Unlock Treatment For Aggressive Lung Cancer



Facebook comments